Case Presentation:
You are in the CCU when you hear an RRT overhead. You arrive at the room to find a 64 year-old male with PMH of HTN, DM, IV drug use, and hyperthyroidism who is currently in the hospital being treated for osteomyelitis of the right foot now s/p right BKA. On further evaluation, the patient is tachycardic with heart rates in the 150-160s with BP 60/40s but is saturating well on room air. On physical exam, the patient is alert and oriented x3 but looks slightly pale. The rest of his exam is notable for a right BKA with current wound vac. Attached is the EKG the nurse obtained right before you walked into the room.
Ask Yourself:
1. What is your initial approach to treating this patient?
2. What rhythm is this patient in?
3. What is your approach for determining different types of tachycardia?
4. What would you like to give this patient now and when they go home?
Background:
In this lesson, we will review atrial fibrillation (AF). AF is the most common type of arrhythmia that affects 1-2% of the population (~50 million people in 2020). This irregularly irregular rhythm is called atrial fibrillation due to the multiple signals being released within the atrium that cause the muscle to fibrillate.
If a patient with AF is having tachycardia, we call this rhythm AF with rapid ventricular rates (RVR). Some patients can have AF with slow ventricular response (SVR) if they have AV nodal conduction disease.
There are more cases of AF now than ever, most likely due to better detection, people living longer, increased cardiovascular disease, and the obesity epidemic. Many studies show that patients with AF have a 1.5-2 fold increased risk of death, as well as increased risk of stroke, cognitive decline, dementia, MI, and heart failure.
Pathophysiology:
From an electrical standpoint, AF is triggered when an ectopic focus of electrical activity, usually in or around the pulmonary veins, sends unorganized waves of electrical signals, causing abnormal atrial contractions.
In this figure, you can see how the pulmonary veins have many areas of ectopy around them (left). As a result, AF ablations include radiofrequency or cryoablation ablation around the entrance to the pulmonary veins (right). In these ablations, a catheter is passed through from the IVC to the right atrium and through the interatrial septum to access the pulmonary veins.
Risk Factors:
Risk factors for AF tend to fall into four main groups:
Worsening cardiac disease
Acute decompensated heart failure is a major cause of AF. The more fluid overloaded patients are, the more atrial stretch they get and the more likely they are to develop AF. The worse the cardiac output, the more likely the patients are to become tachycardic in order to help increase perfusion (HRxSV=CO)
Other cardiac issues that can cause AF include pericarditis, myocarditis, ischemia, CAD, or worsening valvular disease. Patients who undergo major cardiac surgery also have an increased risk of developing post-op AF.
. 2. Worsening pulmonary disease
Increased right heart strain in a patient is due to increased pulmonary pressures, and the more atrial stretch and the more likely the patient will go into AF. This can be caused by acute pulmonary disease, hypoxemia, COPD, PE, OSA, and PNA. All patients without clear precipitating reason for AF should undergo sleep study to rule out OSA as a contributing cause for their AF!
3. Increased metabolic stress
A MAJOR cause for this in the hospital is worsening or untreated infection!! These patients may be septic and severely fluid down. The tachycardia is just the patient’s way of trying to compensate! Note: If you give these patients medications that decrease their rates, you may be taking away their bodies ability to compensate. In these instances, you will quickly see them become decompensated.
4. Drug-induced
This is due to the catecholamine surge. Some medications are pro-arrhythmogenic, like dobutamine and norepinephrine. Others include alcohol and illicit drugs, like cocaine and methamphetamine. Also, we see a lot of AF peri-operatively due to the effects of anesthetic drugs.
Other major causes of AF include CKD, thyroid disease, genetics, increased age, smoking, sedentary lifestyle, and obesity.
Interestingly, the new guidelines do NOT include caffeine as a factor that triggers or worsens AF (rated 3: No Benefit).
Once AF is diagnosed, we should focus on assessing stroke risk, modifying any factors that may be exacerbating the AF (such as sleep apnea, heart failure, HTN, thyroid imbalance, obesity etc.), managing symptoms, and decreasing AF burden.
As seen in the picture from the AHA/ACC 2023 AF guidelines, we should screen patients from HEAD 2 TOES to help manage symptoms and decrease AF burden.
When AF is diagnosed, all patients should have their stroke risk assessed, modifiable risk factors optimized, and symptoms treated (with rate vs rhythm control). All of these decisions should be made through shared decision making with the patient. Use the HEAD-TOES pneumonic to remember all the modifiable risk factors, which include heart failure, exercise, atrial hypertension, diabetes, tobacco use, obesity, ethanol use, and sleep apnea.
Modifiable risk factors (HEAD-TOES):
Heart failure
Treat with GDMT (RACE 3–treating pt’s for AF and early HF improved sinus rhythm)
Exercise
210 minutes per week to reduce AF symptoms and burden
Arterial blood pressure
Optimal blood pressure control is recommended to reduce AF recurrence and related cardiovascular events
Diabetes
Tobacco use
Obesity
10% weight loss recommended when BMI >27 kg/m2
Ethanol
Specifically for patients who are seeking a rhythm-control strategy
Sleep disorders
It may be reasonable to screen for OSA; however, it is uncertain how related the treatment of sleep disorders helps to maintain sinus rhythm.
Classification:
The 2023 AHA/ACC AF guidelines step away from classifying AF based on arrhythmia duration, which tended to focus on AF after it was already diagnosed. Now, the guidelines recommend seeing AF as a progressive disease, which allows for risk prevention, modification, and screening for high risk patients who have yet to develop AF.
The picture below from the AHA/ACC 2023 AF guidelines show how AF should be classified on a continuum from those patients who are at risk for AF to those who have permanent AF.
Once patients are diagnosed with AF, they can be classified as follows:
→ Paroxysmal Atrial Fibrillation
-Episode lasts less than 7 days.
-Left atrial size is usually normal
-Left atrial scar burden is usually low.
-Antiarrhythmic drugs are often effective.
-Cardioversion can be first line therapy. Patients must be in AF in order for them to be cardioverted! Many times, patients can go back into sinus rhythm and will thus not need cardioversion.
→ Persistent Atrial Fibrillation
-Episode last more than 7 days
-Left atrial size is usually mild to severely enlarged.
-Left atrial scar burden is usually moderate.
-Antiarrhythmic drugs are not as effective.
-Ablation as first-line therapy is appropriate, but it is usually only offered after anti-arrhythmic drug failure. ~75-85% of ablations are successful. The longer the patient has been in AF, the more likely it is they will require repeat ablations due to persistence of the AF.
→ Long standing persistent Atrial Fibrillation:
-The patient and their cardiologist have decided that the patient will remain in AF. This can be due to recurrence of AF despite multiple attempts at sinus rhythm or the patient not being a candidate for further ablation or antiarrhythmic therapies.
- Left atrial size is usually severely enlarged.
- Left atrial scar burden is usually high.
- Usually refractory to anti-arrhythmic drugs.
-Ablation unlikely to be successful.
- In these patients, a rate control strategy to prevent uncontrolled rates is employed.
→Permanent Atrial Fibrillation:
- This is the final stage of AF when the patient and physician decide together that the patient will stay in AF with no additional rhythm control therapies
Many times, patients with paroxysmal AF will be sent home with a cardiac monitor to determine how often they are in AF and their baseline heart rates. Any time a patient has an episode of AF > 24 hours, they have a significantly higher stroke risk. In contrast, episodes < 5 minutes are not associated with clinical events.
Note: The longer patients are in AF, the harder it is for them to return to sinus rhythm because of increased scar formation and increase in atrial size. Therefore, it is better to intervene sooner than later!
It’s very important that you document the type of AF the patient has because it helps portray the patient’s burden of AF and how successful the different therapies will be.
Workup:
When a patient is first diagnosed with AF, it is important to order the following:
Basic labs, like CBC, CMP, and TSH. This helps determine if there are any coexiciting disorders, such as CKD, liver dysfunction, or thyroid dysfunction that may impact management. As many of these patients will also be started on anticoagulation, it is important to also get a CBC to understand bleeding risk
EKG will help assess if there are any coexisting atrial arrhythmias
TTE to assess cardiac structure, chamber size, function, and potential valvular pathologies. Strain imaging can even help to assess if there is any underlying infiltrative cardiomyopathy (like amyloid). Assessing LA volume is also crucial as higher volumes correlate with more persistent AF that may return after interventions for rhythm control
Telemetry to assess the patient’s AF burden
Anticoagulation:
All patients with AF are at an increased risk for cardioembolic phenomena due to the concern for clot development in the left atrial appendage (LAA). Anytime a patient develops AF, we must calculate their CHA2DS2-VASc score, which helps us determine their risk for stroke. This figure shows the LAA and where a clot can form. The higher this score, the greater their chance for developing a cardioembolic phenomena. A risk-benefit conversation regarding the need for anticoagulation to prevent any cardioembolic phenomenon balanced with the risk for bleeding should be had with each patient who has AF.
Note: Direct oral anticoagulants (DOACs) like apixaban and xarelto have been shown to be superior and non-inferior to warfarin for treating AF. The only time warfarin should be used is if a patient has moderate-severe rheumatic mitral stenosis or has a mechanical heart valve.
The associated figure depicts the right atrial appendage and shows where the clot forms within the LAA. Due to each patient’s increased risk for cardioembolic phenomena, you must calculate and document their CHA2DS2-VASc score to assess their risk!
This figure from the ACC/AHA 2023 AF guidelines shows how we use both the CHA2DS2-VASc score and the atrial high-rate episode (AHRE– i.e. how long patients are in AF) to determine whether or not we should start anticoagulation. When determining the AHRE burden, we use the longest recorded AF episode.
Patients with contraindications to anticoagulation (i.e. past major bleed, labile INR, intracranial hemorrhage, etc) should be considered for a left atrial appendage occlusion device, AKA a Watchman.
Patients with a CHA2DS2-VASc score of > 2 who are undergoing cardiac surgery should have a surgical LAA occlusion
Note: Per the ACC/AHA guidelines, patients with AF (not including those with recent stroke or mechanical valve) on warfarin or a DOAC who are scheduled for an invasive procedure or surgery should have their anticoagulation held without any bridging.
CHA2DS2-VASc score should be calculated on every AF patient as this will determine the patient’s risk of thromboembolism
There are newer scores, such as ATRIA and GARFIELD-AF that have been shown to modestly improve risk discrimination; however, they have not been validated like CHA2DS2-VASc.
CHA2DS2-VASc is not perfect as it does not include AF duration, obesity, hypertrophic cardiomyopathy, poorly controlled HTN, GFR <45, proteinuria, and enlarged LA volume, all of which increase AF severity and risk of thromboembolism.
** Remember! Risk is based on the CHA2DS2-VASc and NOT duration or pattern (i.e. paroxysmal, persistent, long-standing persistent, or permanent) of AF.
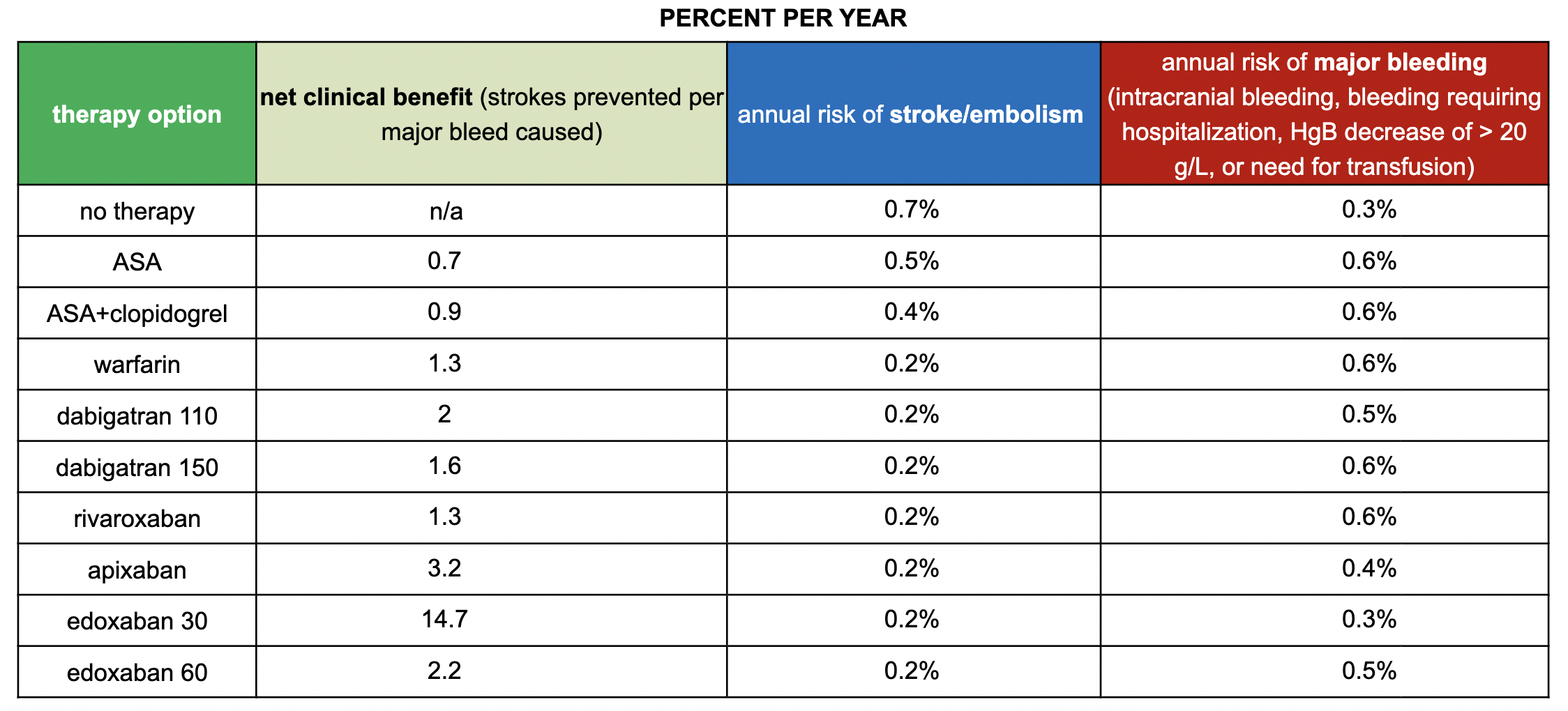
SPARCtool - Stroke Prevention in Atrial Fibrillation Risk Tool
This scoring system can be used to help estimate the risks and benefits of antithrombotic therapy in patients with chronic nonvalvular atrial fibrillation
In the first section, you fill out the patient’s CHA2DS2-VASc Score, which helps determine the patient’s risk for developing a stroke, and the HAS-BLED Score, which shows the patient’s risk for bleeding.
Next, you pick the specific agent the patient is taking.
With this information, the calculator will help generate the net clinical benefit, annual risk of stroke/embolism, and risk of major bleed for each medication.
The age old debate: Rate vs. Rhythm Control?
While the AFFIRM trial showed that there was no difference between rate vs rhythm control, later studies have demonstrated that rhythm control (whether with ablation or medication) was deemed superior for preventing composite cardiovascular outcome events. The Early Treatment for Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET4) showed that early rhythm control in new-onset, symptomatic AF was better than rate control, specifically improving death and stroke at 5 years. The patients from this trial also had better outcomes at 12 months when maintained in sinus rhythm. There are even trials that show that allowing persistent AF may be implicated in early dementia (Bunch, Circulation, 2020)!
Rhythm control should especially be trialed in patients with AF and reduced EF as the heart failure may be due to the AF. If this is true, the EF will improve when the patient is back in sinus rhythm.
In certain patients, catheter ablation is better when compared with antiarrhythmic medications as some of the antiarrhythmic medications can cause potential long-term toxicities. CASTLE-AF showed that catheter ablation actually lead to an absolute reduction in death and hospitalizations in patients with AF and symptomatic heart failure.
Remember: Everyone deserves a chance in sinus rhythm!
Cardioversion:
Also known as a Direct Current Cardioversion (DCCV), this method should be done if patients are hemodynamically unstable. If patients are stable, cardioversion can be an initial rhythm control strategy or it can be used after pharmacologic cardioversion has failed.
If patients have been in AF for > 48 hours, cardioversion should be accompanied by a transesophageal echocardiogram (TEE) to ensure that there is no clot in the left atrial appendage. We do not do a TEE when patients are unstable and when they have reliably been on anticoagulation for at least 3 weeks prior to the cardioversion.
Note: Patients MUST be maintained on anticoagulation for at least 4 weeks after cardioversion even if they remain in sinus rhythm! This is because there is atrial stunning after cardioversion that can cause the patient to go back into AF within the first month after the procedure.
When thinking about AF, the algorithm pictured helps provide a good framework for determining medications for AF. The first point is to assess whether or not the patient is stable. If they are not, SHOCK!
If you have some time, you can choose either rate or rhythm control.
Rate Control:
Ask yourself: Is the patient decompensated or not? If they are, giving them calcium channel blockers (CCB) or beta blockers (BB) will decrease their chronotropy and cause them to become more decompensated. Therefore, if they are decompensated, digoxin or rhythm control might be better.
Rhythm Control:
There are three options:
1. Ablation
2. Synchronized Cardioversion
3. Medications
When thinking about medications, Flecanide and Propafenone are great options if the patient has a structurally normal heart with no CAD history (the CAST trial showed that patients with recent MI who were put on these Class 1c drugs had increased risk for death)
Amiodarone and Dofetilide are great options for patients with reduced EF and for those with normal hearts and no CAD who fail Class 1c drugs.
Sotalol is the last line
Ablation:
In patients with symptomatic AF, catheter ablation is recommended when they are not candidates for antiarrhythmic drugs, when the drugs have not been effective or tolerated, or when the patient prefers rhythm control.
Pulmonary vein isolation (PVI) ablation is recommended as the primary lesion in patients who are going to get an ablation for AF (see pictures below).
Recurrence of AF after ablation occurs in 30-40% of patients after one year. Many times after ablation, AF can return. According to the 2023 ACC / AHA / ACCP / HRS AF Guidelines, patients with recurrent symtpomatic AF should have repeat catheter ablation or be started on antiarrhythmic drugs (Class 1 recommendation).
Patients who are on anticoagulation prior to ablation, whether that be warfarin or a DOAC, should have these medications continued and NOT STOPPED up to and through ablation (see table below).
After ablation, patients need to remain on anticoagulation for at least 3 months as thromboembolic and stroke risk increases during this time period due to the instrumentation and inflammation from the ablation. Overall continuation of anticoagulation after these 3 months will depend on the individual patient’s CHA2DS2-VASc score.
Many AF ablations focus on ablating the tissue around the pulmonary veins. The pictures above show how cryoablation or pulsed field ablation can be directed at the entrance of the pulmonary veins.
In contrast to ablating the entrance to the pulmonary veins, the pictures above show another approach that involves ablating the tissue around the pulmonary veins in a wide antral circumferential ablation (WACA). themselves to fully prevent the ectopic signals that originate in the pulmonary veins from traveling to the heart (as seen by the purple lines).
The table above, taken from the 2023 ACC / AHA / ACCP / HRS AF Guidelines, helps to elaborate what to do with the patient’s anticoagulation during and after ablation. Unlike many other procedures in medicine, we actually continue patients’ anticoagulation during the AF ablation.
In the figure above, you can see the progression of AF and the effects on the heart. When the patient is in AF, the less atrial kick they have. This, in turn, causes increased filling pressures. Overtime, this causes increased atrial and ventricular chamber size, as well as decreased systolic function, cardiac output, and mitral insufficiency.
TTE: reduced EF with macroscopic extracellular matrix changes and biventricular dilation with concurrent mitral insufficiency due to mitral annular dilation.
Additionally, baseline tachycardia can impair diastolic and systolic function and increase LV filling pressures, which can precipitate functional mitral regurgitation, increase left atrial size, and ultimately worsen AF.
The higher the rates, the less filling time the heart has, and the worse the cardiac output!
Complications of Uncontrolled AF:
1. Arrhythmia induced cardiomyopathy:
Heart failure and AF go hand in hand as one can cause and worsen the other. Patients with heart failure can develop AF– and vice versa. Sometimes, it is difficult to tell which came first and caused the other. AF can cause worsening EF as sustained tachyarrhythmias can cause changes in the ventricular structure and function over time. The higher the heart rates, the more stress there is on the heart, the less atrial kick there is, the more AV dyssynchrony there is, and the worse the overall cardiac output becomes. increased rates and duration are inversely proportional to worsening EF.
Interestingly, if AF is the cause of the heart failure, treatment of the AF should also treat the heart failure. Although EF may return to normal, these patients are at high risks of AF recurrence due to underlying structural changes that make them more susceptible to arrhythmia.
This figure shows the cycle of AF and heart failure and how they can each cause and worsen the other. The more overloaded a patient is with heart failure, the more atrial stretch there is. As the atrium get stretched, this increases the patient’s risk for developing AF. When patients have AF, they lose their atrial kicks, which ultimately cause ventricular irregularity, decreased cardiac output, and increased filling pressures. Overtime, this causes both atrial and ventricular dilation, which weakens the heart and worsens the patient’s heart failure.
2. Cardioembolic stroke:
Blood stasis from loss of synchronous atrial contraction promote the development of thrombus, especially in the left atrial appendage, increasing risks of strokes. Recurrent embolic events can develop and cause severe neurologic sequelae. Remember: We only worry about a clot forming in the left atrial appendage (LAA) after the patient has been in AF for >48 hours. If we are not sure how long the patient has been in AF, we MUST check the LAA before mechanically (via cardioversion) or medically (through antiarrhythmics) converting patients into sinus rhythm.
Guideline directed medical therapy (GDMT): Patients with decreased EF due to AF should be rhythm controlled and given GDMT indefinitely due to underlying ventricular remodeling that puts their heart at higher risk for recurrence
Back to the Case:
1. What is your initial approach to treating this patient?
Determine whether the patient is stable. Regardless, make sure you put pads on the patient! In this case, the patient is in AF. Remember: AF is usually caused by something else, such as sepsis, fluid overload, and hypovolemia. DO NOT JUST GIVE BETA BLOCKERS AS THIS CAN CAUSE RAPID DETERIORATION OF THE PATIENT, especially in patients with ADHF.
2. What rhythm is this patient in?
We can see here from the lack of organized atrial activity (i.e. p waves) and irregularly irregular heartbeat that the patient is in AF. It is crucial that you get an EKG so you can accurately determine if there are p waves!! Many times, patients can have irregularly irregular heart rhythms but that can be due to OTHER things other than AF, such as heart block, premature atrial / ventricular contractions, and multifocal atrial tachycardia (i.e. there are multiple different origins of atrial activity and you will see multiple types of p waves).
3. What is your approach for determining different types of tachycardia?
Look at a 12-lead EKG and telemetry!
4. What would you like to give this patient now and when they go home?
In this case, the patient is extremely hypotensive in the setting of recent infection and surgery. The first step would to be ensure that you are not missing any underlying pathology, such as sepsis, hypovolemia, fluid overload (i.e. acute decompensated heart failure), or increased catecholamine surge. Before giving ANY rate control agents, you can try putting the patient in trendelenburg or raising their legs up to see if they will be fluid responsive. If their blood pressure increases, try giving fluids first! If you are going to give a rate control agent, start with a SHORT ACTING AGENT, such as esmolol.
Many times, AF may be limited to the hospital. In the event that this is new AF, make sure to calculate the CHA2DS2-VASc to determine if the patient needs anticoagulation. If the patient is persistently in AF, he may be a good candidate for cardioversion or antiarrhythmic therapy (depending on whether or not he has underlying cardiac disease).
Further Learning:
Resident Responsibilities
Evaluate for risk factors and correct reversible causes. Do NOT just focus on the rate!!! Remember, if you slow down a patient’s heart rate, you may be taking away their only their compensation
Always check telemetry as most cases of A fib or A flutter are abrupt changes with minimal HR variability and flat strips on telemetry.
Remember to classify your AF as either paroxysmal, persistent, or long-standing persistent!!
Always check a 12 lead EKG and evaluate if there is presence of F waves or a pattern between QRS complexes that could justify A flutter with 2:1, 3:1, or 4:1 AV conduction.
Always calculate CHA2DS2-VASc Score on every patient and start AC if warranted. If patient is on antiplatelet regimen discussion about the potential risks of GI bleed vs benefits needs to be discussed.
Consult cardiology for evaluation of cardioversion and whether catheter ablation can be pursued especially in cases of A flutter with minimal response to rate and rhythm control. A TEE must be done if a patient was in AF for > 48 hours. Any patient who undergoes a cardioversion should be continued on anticoagulation for at least 4 weeks due to atrial stunning and high risk of clot formation.
Anticoagulation is absolutely necessary for 1 month post cardioversion and 3 months post ablation even if the patient remains in sinus rhythm due to concern for atrial stunning and return of the AF.
Further Reading
CASTLE-AF trial
o Showed patients with paroxysmal A fib or persistent A fib with HFrEF who underwent ablation had less overall mortality and reduced hospitalized in comparison to patient who only underwent rate/rhythm control.
CABANA trial
o Evaluated the effect of catheter ablation vs medical rate/rhythm control on mortality, stroke, bleeding, and cardiac arrest in patients with A fib, which showed no significant reduction in death, disabling stroke, serious bleeding, or cardiac arrest with ablation in comparison to medical rate/rhythm control.
EAST-AFNET trial
o This one evaluated the effects of early rhythm control strategies in overall death from heart disease, strokes, or hospitalizations with worse HF. It showed early rhythm control had a lower risk of death from CV causes and strokes.
2023 ACC / AHA / ACCP / HRS AF Guidelines
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001193#d16556e1
Attending Pearls
Remember to document type of AF!
Any time you list AF as a problem, make sure to include the patient’s CHA2DS2-VASc Score!
If a patient is NOT on anticoagulation, make sure you document very clearly why this decision was made. Remember— starting or stopping anticoagulation is a shared decision between the team and the patient.
When determining rate vs rhythm control, it’s important to take into account any structural changes that may have occurred because of the AF, such as atrial dilation or even decreased EF. The bigger the atria, the harder it is for the patient to maintain sinus rhythm.
How’d we do?
The following individuals contributed to this topic: Richard Nieves Santiago, MD; Mark McCauley, MD; Isaac Whitman, MD; Stephanie Dwyer, PharmD; Jinjoo Chung, PharmD
Resources
Joglar, José A., et al. “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.” Journal of the American College of Cardiology, vol. 83, no. 1, 2024, pp. 109–279, https://doi.org/10.1016/j.jacc.2023.08.017.
Joshua A. Keefe, Rebecca Garber, Mark D. McCauley, Xander H.T. Wehrens, Tachycardia and Atrial Fibrillation-Related Cardiomyopathies: Potential Mechanisms and Current Therapies, JACC: Heart Failure 2024,
Loscalzo, J. (2022). Harrison's principles of internal medicine, twenty-First Edition (vol. 1 & vol. 2). McGraw-Hill Education.
Chiu, A., Kasper, M., Rimmer, J., Donnelly, M., Chen, Y., Chau, C., Sidow, L., & Ash, A. (2017). Remote Management of Atrial Fibrillation: A case report. Clinical Practice and Cases in Emergency Medicine, 1(3), 242–245. https://doi.org/10.5811/cpcem.2017.4.33539
Sabatine, M. S. (2023). Pocket medicine: The Massachusetts General Hospital Handbook of Internal Medicine. Wolters Kluwer.
Flautt, T., & Valderrábano, M. (2021). Recent clinical trials in atrial fibrillation. Current Opinion in Cardiology, 36(6), 798–802. https://doi.org/10.1097/hco.0000000000000914
Dobariya V, Ezeh E, Suliman MS, Singh D, Teka S. Unusual Presentation of Atrial Flutter With Slow Ventricular Response. Cureus. 2021 Jun 21;13(6):e15801. doi: 10.7759/cureus.15801. PMID: 34306869; PMCID: PMC8294030.
Nesheiwat Z, Goyal A, Jagtap M. Atrial Fibrillation. [Updated 2022 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526072/
Mayo Foundation for Medical Education and Research. (2021, October 19). Atrial fibrillation. Mayo Clinic. Retrieved May 1, 2023, from https://www.mayoclinic.org/diseases-conditions/atrial-fibrillation/symptoms-causes/syc-20350624 - Image.
YouTube. (2022). Telemetry Tips - Atrial Flutter, Atrial Tachycardia. YouTube. Retrieved May 1, 2023, from https://www.youtube.com/watch?v=BbLYOcgdPb0.
YouTube. (2014). Intro to Ekg Interpretation - Practicing Tachyarrhythmia Identification. YouTube. Retrieved May 1, 2023, from https://www.youtube.com/watch?v=NULY6Ecj6gA.
Organization, Healio, & ImageObject. (2017, August 2). Left atrial appendage thrombus. Left Atrial Appendage Thrombus | Learn the Heart. Retrieved May 1, 2023, from https://www.healio.com/cardiology/learn-the-heart/cardiology-review/topic-reviews/atrial-appendage-thrombus Image.
Wikimedia Foundation. (2023, January 30). Atrial flutter. Wikipedia. Retrieved May 1, 2023, from https://en.wikipedia.org/wiki/Atrial_flutter- Image.
Identifying and treating atrial fibrillation (AFIB or AF). ACLS, PALS, and BLS Certification Online. (n.d.). Retrieved May 1, 2023, from https://www.aclsonline.us/rhythms/atrial-fibrillation/ -Image.
Martin, P. (2018, December 12). Atrial flutter. ACLS Wiki. Retrieved May 1, 2023, from https://www.proacls.com/wiki/ekg-rhythms/atrial-flutter/ - image.