Case Presentation:
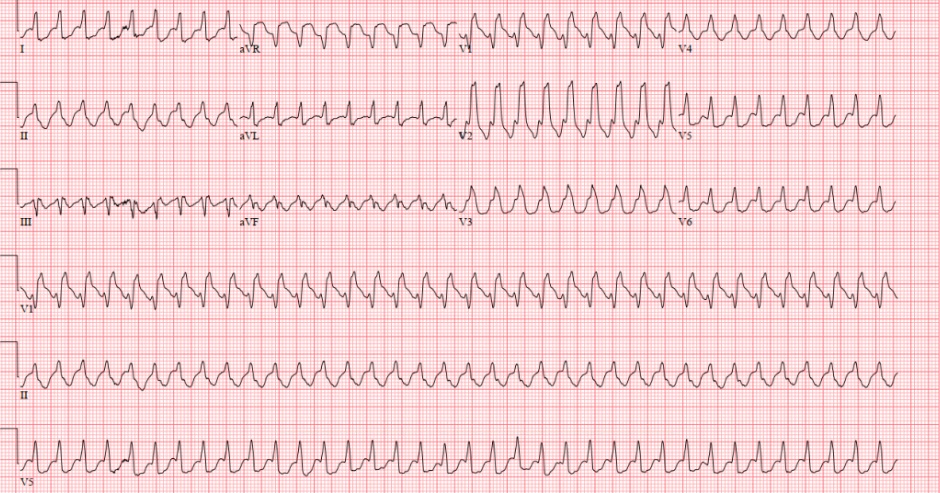
You are working overnight in the emergency room when a 65-year-old male with ischemic cardiomyopathy with recovered ejection fraction (EF) and coronary artery disease (CAD) s/p drug eluting stents (DES) to the right coronary artery and left circumflex artery presents with acute onset shortness of breath and palpitations.
Triage Vitals:
HR: 150 BP: 130/80 Temp: 98.4 SpO2: 95% on room air. RR: 30
Figure 1
Ask Yourself:
What is the most important next step in management?
What blood tests may be helpful to guide treatment?
What arrhythmias are on your differential?
How would your management change if the patient had a known right bundle branch block (RBBB)?
In this lesson, we will be focusing on wide complex tachycardias:
Figure 2
Figure 2 represents how you should think about tachycardias. When the patient is not stable, always do synchronized cardioversion. If the patient is stable stable and the QRS complexes are wide, you must determine whether or not the tachycardia is coming from or above the ventricle.
When looking at the EKG, these are the questions you must ask yourself:
Is the patient stable?
Is the complex narrow (<120ms, i.e 3 little boxes) or wide (>120 ms)?
Is the rhythm originating from above the ventricles (i.e. supraventricular, SVT) or in the ventricles?
____
If the tachyarrhythmia is stable, meaning there are no signs or symptoms of end organ damage, then further workup for diagnosis and administration of medications can be trialed. If the tachyarrhythmia is unstable, then synchronized cardioversion is performed.
Regardless of which pathway is followed:
Remember to establish IV access
Get the patient on the monitor and frequent monitor the patient’s BP!
Look at the EKG that came before, during, and after the tachycardia
You can also look at telemetry to see the graphic trends and what may have preceded the episode!
What Causes Wide QRS Complexes?
Figure 3
Figure 3 demonstrates the normal pathway of conduction as the electrical signal goes from the Sinoatrial (SA) node to the Atrioventricular (AV) node and down the Bundle of His to the right and left bundle branches. This coordinated electrical activity results in a narrow complex QRS (<120 msec or 3 little boxes).
Figure 4
In Figure 4, we see that there is a block in the right bundle (seen by the purple X). As a result, the signal goes from the SA node to the AV node and down the Bundle of His; however, the signal is only able to delivered via the left bundle. Once the left bundle activates the myocardium on the left side of the heart, the signal travels from the left side of the heart to the right side of the heart via the myocardium (as depicted through the large green arrow beneath the heart going from the left side of the heart to the right side). The fact that both sides of the ventricle are not activated at the same time makes for a prolonged signal distribution and thus widens the QRS (seen above as QRS > 120 msec).
What is SVT with aberrancy? What about SVT with an accessory pathway?
Figure 5
Figure 5 shows an SVT with aberrancy. Here you can see the signal going down the left bundle and then moving toward the right side of the heart, mimicking a right bundle branch block. This occurs because the right bundle is too weak to keep up with the higher heart rates. Therefore, the signal can only go down the left bundle.
SVT with aberrancy means that you have a cardiac rhythm that is originating from above the ventricles (i.e. SVT) but there is aberrant conduction, meaning that the signal is only going down one of the bundle branches (Figure 5). This is due to rate dependent decremental conduction, meaning that one of the bundle branches is too weak to keep up with the fast heart rates. As a result, the signal only goes down the stronger bundle branch, which ultimately creates a wide complex tachycardia since both bundles are not able to activate at the same time. However, since the weaker bundle can keep up when the patient is not tachycardic, slower heart rates will still produce a narrow complex. You can also think of SVT with aberrancy as a rate dependent bundle branch block.
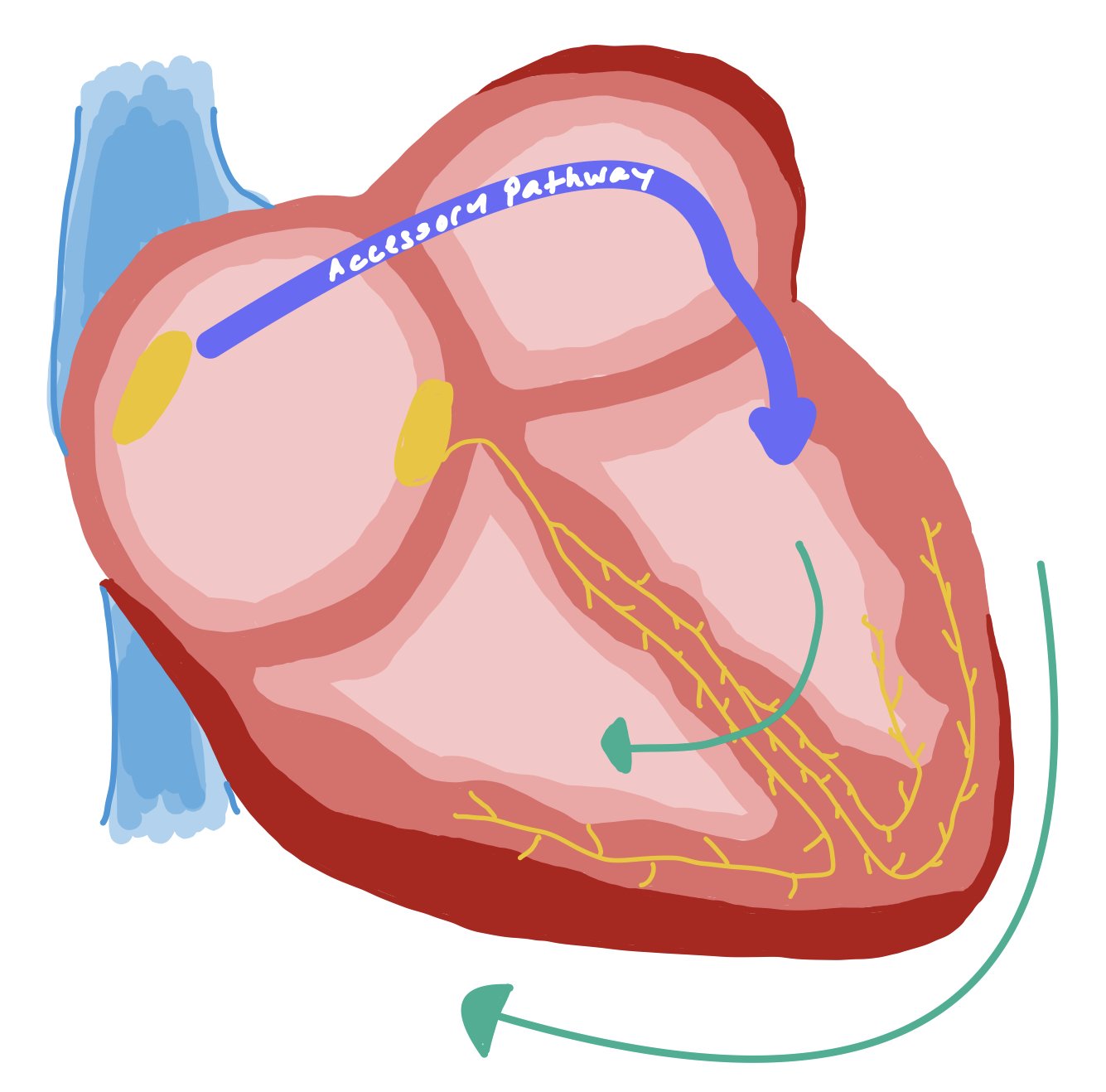
This is in contrast to SVT with an accessory pathway (Figure 6). Instead of the QRS being wide because the signal is only going down a specific bundle branch, it is actually wider because the accessory pathway bypasses the AV nodal system and activates on side of the myocardium on its own. Accessory pathway conduction leads to non-fast pathway activation of the ventricle, thus creating an SVT.
Figure 6
Figure 6 demonstrates an SVT with an accessory pathway. This image demonstrates how there is an extra pathway that activates only one side of the heart. As a result, the signal needs to propagate to the other side of the heart, causing a wider QRS.
Unlike SVT with aberrancy, VT may have:
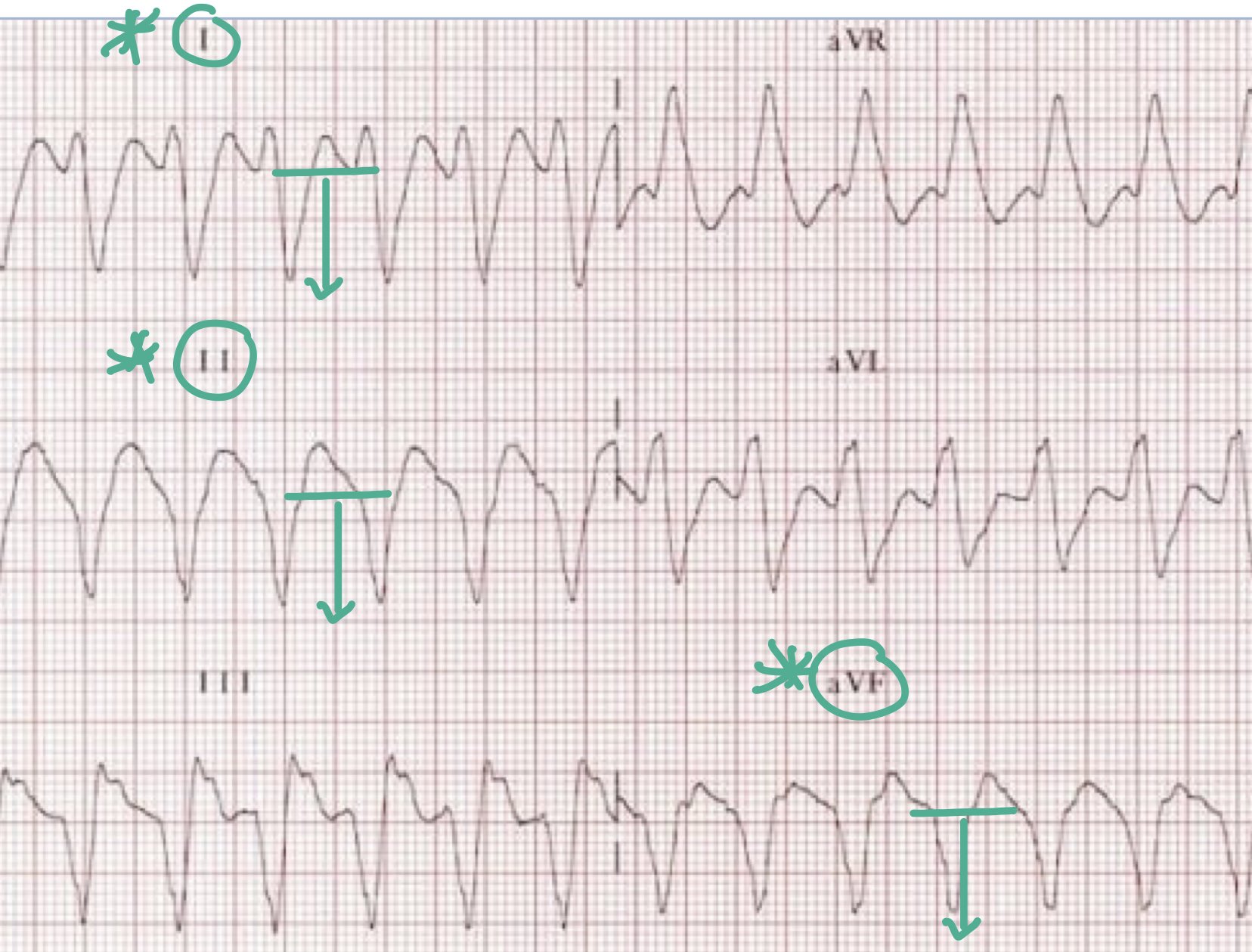
1. A northwest or extreme right axis deviation, seen by negative complexes in leads I, II, and aVF (Figure 7)
Figure 7
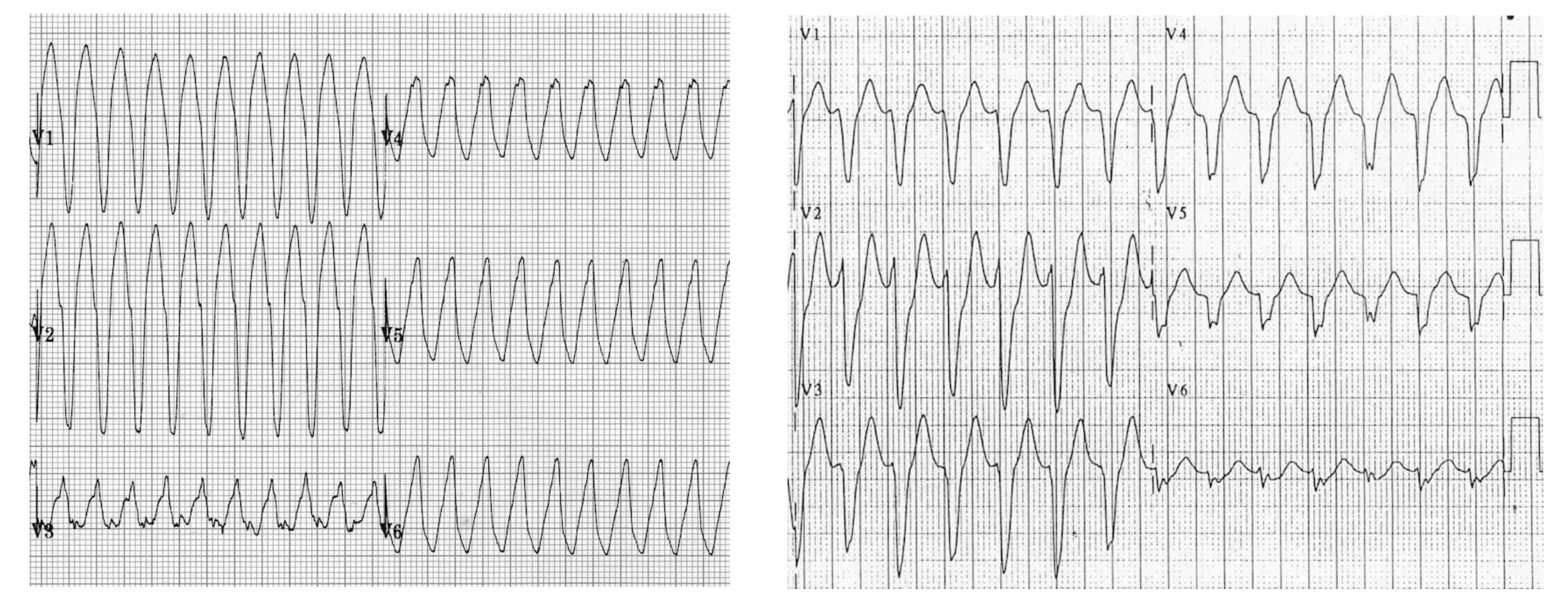
2. Positive or negative concordance, seen by all complexes pointing up (Figure 8a) or down (Figure 8b)
Figure 8a
Figure 8b
3. Atrioventricular (AV) dissociation, seen by P waves that don’t match up with the QRS complexes. In VT AV dissociation, there are more V:A complexes, i.e. more QRS complexes to p waves as ventricles are running the show and p waves are not conducted (Figure 9, blue circles).
4. Rsr’ seen in V1. In other words, the left “bunny ear” is higher than the right (Figure 9, red arrow)
Figure 9
What is a capture beat? What about a fusion beat?
A capture beat occurs when the normal atrial conduction gets conducted through the ventricle. Figure 10 demonstrates how the atrial activity (shown in green) gets conducted through the ventricular activity (shown in red).
In contrast, a fusion beat will be produced when both the atrial and ventricular impulses conduct at the same time and fuse together. Therefore, a fusion beat is the product of the normal conduction from the atrium plus the ventricular activity. Figure 11 shows the ventricular beats (shown in red), the atrial beats (shown in green), and the product of them fusing together to form a fusion beat (shown in purple).
*Presence of either a capture or fusion beat suggests the wide complex tachycardia is ventricular in origin!*
How do we differentiate and treat the different types of VT?
Monomorphic VT – This is usually due to scar formation that can occur after ischemia. The scar acts as a nidus that can induce VT when an electrical signal passes around it. If patients have a high burden of VT, they can go to the EP lab to have the scar ablated (i.e. burned) so that the tissue is no longer electrically active.
Polymorphic VT due to prolonged QTc (aka Torsades de Pointes) – In this case, a premature ventricular complex (PVC) occurs during the prolonged QTc (seen by the red arrow). When this happens, the patient goes into polymorphic VT. This is also known as the R on T phenomenon and why there is so much emphasis placed on ensuring the QTc does not get too prolonged.
Polymorphic VT – If this is NOT due to prolonged QTc, this patient MUST BE TAKEN TO THE CATH LAB because this is due to ischemia until proven otherwise.
Questions to ask yourself:
Does the wide complex look similar to the patient’s baseline? The more the EKG looks like the patient’s baseline, the more likely it is NOT VT.
Is there a fast or slow upsloping of the QRS complex? Slow upsloping is more likely VT (figure 12a), while fast upsloping (12b) is more likely an SVT variant or a tachycardia with underlying bundle branch block.
Is there a 1:1 conduction of P waves to QRS complexes? If this is true, it is NOT VT.
Do you see any fusion or capture beats? Seeing either beat makes VT more likely.
What if someone has a known bundle branch block?
Figure 13, part of the EKG from the beginning of the case, shows a fast upsloping of the QRS, suggesting we are dealing with an SVT. If you look closely, you may also be able to see buried P waves. Since we do not see a NW axis, concordance, AV dissociation, fusion or capture beats, this EKG is consistent with an SVT rather than VT.
What do you do if someone has an ICD?
All defibrillators have the ability to rapidly pace the person out of VT, which is known as anti-tachycardic pacing (ATP). Afterall, many times people have defibrillators placed for primary prevention for VT. Many times, patients go into asymptomatic VT and never even know that the ATP setting on their device terminated their VT. During device interrogations, we can see how often the ATP setting was activated (figure 14).
If patients go into unstable VT while they are in the hospitals, you should use the device magnet to turn off the device so that you can do ACLS. We do this because device can create artifact and make it difficult to know which rhythm the patient is actually in.
If a patient is being ventricularly paced by their device (seen by the pacer spike), this will mimic a bundle branch block as the activated myocardium from the device lead needs to travel to activate the other parts of the heart (figure 15).
Figure 14, courtesy of ResearchGate via B. Swackhamer, shows how a VT is terminated via anti-tachycardic pacing and allows the patient to return to sinus rhythm.
Figure 15 shows a pacer wire in the right ventricle. In the associated EKG, you can see the tiny pacer spikes (as seen by the green arrows) and how this widens the QRS complex.
Pharmacotherapies for Wide-Complex Tachycardias
Lidocaine
Class IB antiarrhythmic (blocks Na channels during resting/inactive state; shortens QTc interval)
Dose: 0.5-1 mg/min IV infusion, may titrate up to max 4 mg/min
Administration tips
Start at lower rate in patients with HF, hepatic/renal impairment, or shock states
Side effects
Toxicity associated with levels > 6 mcg/mL
Early symptoms: facial/oral numbness, tongue paresthesia, LH/dizziness
Visual/auditory disturbances, blurred vision, agitation, muscle twitches, seizures
Severe: CNS depression, unconsciousness and coma
Monitoring
If using for >24h duration, monitor drug level (therapeutic levels 1.5-5 mcg/mL)
Amiodarone
Class III antiarrhythmic (blocks K channels), with additional beta-blocking and Na/Ca channel antagonism
Dose: 150 mg IVPB, followed by IV infusion 1 mg/min x 6 hrs, then 0.5 mg/min x 18 hrs
Administration tips
May complete 8-10 g load with PO amiodarone once arrhythmia suppressed
Side effects (can be severe with prolonged use)
Hyper/hypothyroidism
Hepatotoxicity (fibrosis, cirrhosis, cholestasis)
Pulmonary toxicity (pneumonitis, PAH, pulmonary fibrosis, chronic eosinophilic pneumonia)
Optic neuropathy
Despite QTc prolongation, may be least proarrhythmic in patients with structural heart disease
Monitoring
LFTs, TFTs, CXR, PFTs
Procainamide
Class IA antiarrhythmic (blocks Na channels; decreases conduction velocity, prolongs QTc)
Recommended agent in SVT w/ known accessory pathways
Dose: IV 10-17 mg/kg admin at 20-50 mg/min (or in increments of 100 mg Q5min up to 17 mg/kg)
Administration tips
If starting maintenance dosing (IV 1-5 mg/min or PO 500-1250 mg Q6h) after arrhythmia suppression, requires renal/hepatic dose adjustments and CBC monitoring
Side effects
QTc and QRS prolongation
Monitoring
Stop infusion if arrhythmia terminates during bolus, patient becomes hypotensive, or QTc/QRS widens >50%
Pharmacotherapies for SVTs
Adenosine
AV nodal blocker; interrupts re-entry pathways through AV node
Dose: 6 mg rapid IVP x 1, may repeat 12 mg x 2 (some data to support higher 18 mg as 3rd dose)
Administration tips:
Give via 2-way stopcock (adenosine first, quickly followed by NS flush) OR dilute adenosine with 15-20 mL NS into a single syringe to reduce administration delays/errors
Administer into most proximally available peripheral IV
Very short half-life (<10 sec)
Contraindication(s): Patients with accessory pathways (e.g., Wolff-Parkinson White), where AV nodal blocking will further promote conduction down the AP and can cause ventricular arrhythmias
Non-DHP Calcium Channel Blockers
Block L-type calcium channels to delay AV nodal firing
Reasonable 1st line therapy for SVT without pre-excitation
Pros:
Requires less coordination for administration than adenosine
Lower incidence of patient discomfort (dyspnea, chest tightness, flushing) compared to adenosine
2017 Cochrane review showed similar conversion rates to NSR compared to adenosine, without significant differences in ADRs1
Cons:
May potentially be associated with more hypotension
Slower onset (3-5 min) compared to adenosine
Longer duration of action (2-5 hrs) compared to adenosine
Not preferred in patients who are HDUS
Common agents:
Diltiazem:
Dose: IV bolus 0.25 mg/kg (~20 mg) over 2 min, may repeat 0.35 mg/kg (~25 mg) IVP at 15 min
Verapamil
Dose: IV bolus 2.5-5 mg over 2 min, may repeat with 5-10 mg at 15 min
Procainamide
Recommended agent in SVT w/ known accessory pathways
Class IA antiarrhythmic (blocks Na channels; decreases conduction velocity, prolongs QTc)
Dose: IV 10-17 mg/kg admin at 20-50 mg/min (or in increments of 100 mg Q5min up to 17 mg/kg)
If starting maintenance dosing (IV 1-5 mg/min or PO 500-1250 mg Q6h) after arrhythmia suppression, requires renal/hepatic dose adjustments and CBC monitoring
Side Effects:
QTc and QRS prolongation
Monitoring:
Stop infusion if arrhythmia terminates during bolus, patient becomes hypotensive, or QTc/QRS widens >50%
Back to the Case…
You are working overnight in the emergency room when a 65-year-old male with ischemic cardiomyopathy with recovered ejection fraction (EF) and coronary artery disease (CAD) s/p drug eluting stents (DES) to the right coronary artery and left circumflex artery presents with acute onset shortness of breath and palpitations.
Ask Yourself Answer Key:
What is the most important next step in management?
Once you ascertain that the patient is stable and has pads on (just in case), the most important part of diagnosing the tachyarrhythmia is getting a 12 lead EKG and looking at telemetry so you can look at tachyarrhythmia onset, length of duration, cessation, and graphic trends. Comparing the current EKG to past EKGs can also be very helpful as sometimes baseline wide QRS complexes can be mistaken for VT when they become tachycardic. Remember that patients with structural heart disease and history of ischemia are more likely to have VT; however, you still must rule out other arrhythmias as well.
What blood tests may be helpful to guide treatment?
Getting a BMP to assess electrolytes, especially potassium and magnesium, is crucial as electrolyte abnormalities can precipitate cardiac arrhythmias, especially VT. If patients are having longer runs of VT or new polymorphic VT, get a troponin because cardiac ischemia can greatly precipitate these rhythms! Just note that troponin elevations can be difficult to interpret as due to new ischemia vs the demand from the arrhythmia itself.
What arrhythmias are on your differential?
After confirming a regular, wide-complex tachycardia on EKG (QRS > 120 msec), the differential includes VT, regular sinus tachycardia in patients with baseline bundle branch blocks, SVT with aberrancy.
How would your management change if the patient had a known right bundle branch block (RBBB)?
The overall management should remain the same. Make sure to compare the current EKG to previous and to look for VT specific criteria, such as a NW access, positive / negative concordance, AV dissociation, and a Rsr’ in lead V1. Additional features, such as fast vs slow upsloping of R wave, presence of pacing spikes (which might make the QRS look wider), and rate of the tachycardia (< 140 beats per minute less likely VT) can also be used to help rule in or out VT.
Further Learning
Resident Responsibilities
If you’re seeing a wide complex tachycardia, make sure the patient is stable (i.e. the patient has a pulse, not hypotensive, alert and oriented)
During your initial assessment, make sure the patient has appropriate IV access, pads on, and an EKG
Assume any new wide complex tachycardia is VT until proven otherwise, especially if they have known structural heart disease!
If available, get a prior EKG to compare to the current one to in order to assess for any underlying abnormality, such as a known bundle branch block.
Looking at telemetry to help assess arrhythmia onset is crucial to help determine etiology and type of arrhythmia.
Features that make VT more likely include new NW access, positive / negative concordance, AV dissociation, Rsr’ in V1, and fusion and capture beats.
If you are seeing new polymorphic VT, look to see if it was precipitated by a prolonged QTc. If not, this is ischemia until proven otherwise and the patient MUST go to the cath lab.
Further Reading
The AVID trial: In patients with NYHA class II / III and LVEF ≤ 35% who survived sudden cardiac death or hemodynamically unstable VT, antiarrhythmic medication therapy (primarily amiodarone) had no favorable effect on survival when compared with single-lead ICD therapy, which reduced overall mortality by 23%.
The DANISH Trial: In patients with nonischemic systolic heart failure, an ICD reduced sudden cardiac death from 8.4% to 4.3%; however, there was no difference in all- cause mortality.
Attending Pearls
The reason prolonged QTc can cause torsades is because of an R on T phenomenon, which essentially means that a PVC occurs during the T wave. Ensuring we are not giving any QTc prolonging medications and that patients are repleted for Mg >2 and K >4 helps prevent this phenomena.
If patients are in VT and have a defibrillator, the defibrillator will try to pace them out of that rhythm via antitachycardic pacing (ATP). If this doesn’t work, the device will shock the patient. We interrogate device to see how often the patient is requiring ATP. If a patient has a large burden of ATP, this is usually due to scarring secondary to ischemia. In these cases, patients can go to the EP lab and have the scar tissue burned so that these foci no longer conduct electricity and cause VT when the signal travels through it.
How’d we do?
The following individuals contributed to this topic: Rachel Brett, DO, Mayank Kansal, MD, Stephanie Dwyer, PharmD, Jinjoo Chung, PharmD