Case Presentation:
The ED calls to admit a 45 year old male with PMH of ETOH-related cirrhosis and thrombocytopenia with a new presentation of worsening SOB, abdominal distention, and bilateral lower extremity edema.
Ask Yourself:
Questions:
What do you think is causing the patient’s new edema and SOB?
What labs, tests, and images should you order?
How might the patient having a fever complicate his presentation?
What do you think about his bedside POCUS?
How can we best improve the shortness of breath?
Vitals: Afebrile. HR 100s, BP 90s/60s, saturating 90% on 5L
Labs: BNP 500, HS troponin 110→ 470. Lactate 3. CBC with hgb 8.5 (baseline 10), PLT 70 (baseline 150), WBC 7. LFTS with elevated Alk phos 180s, AST 50s, ALT 30s, total bilirubin 4. INR 2.6.
Imaging:
CXR: Notable cardiomegaly with pulmonary vascular congestion.
CTPE: Negative for PE. However, notable distal vascular dilatation with subpleural telangiectasias
Bedside POCUS: hyperdynamic heart with EF estimated at 40%.
Here you can see a coronal chest CT of a patient with a similar pathology. Picture courtesy of Radiopaedia
Background:
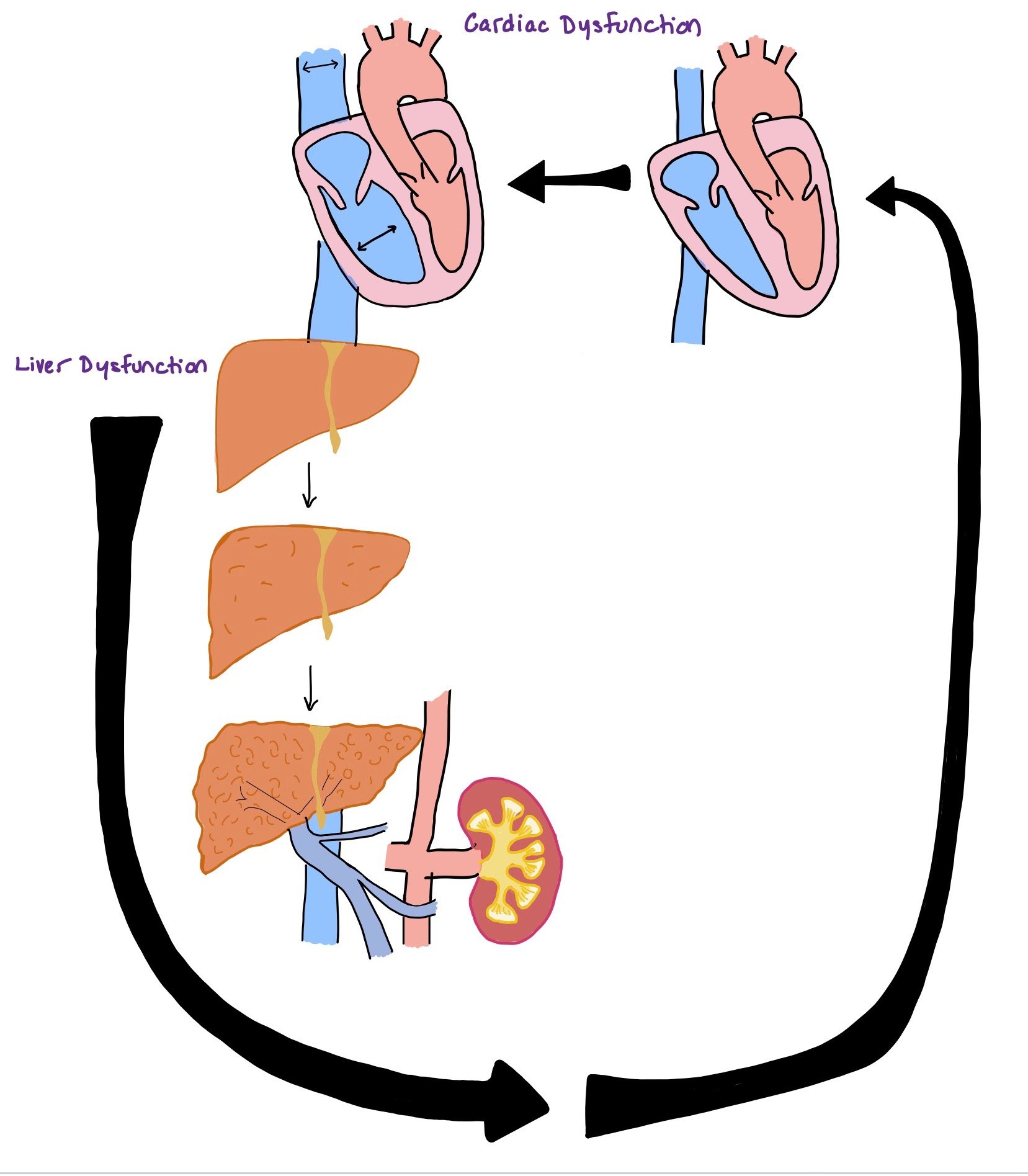
Cardiac and liver dysfunction can be very interrelated as dysfunction in one can lead to dysfunction in the other (as seen in the picture).
Approximately half of the patients with advanced cirrhosis have cardiac dysfunction. Alcoholism and hemochromatosis, two major causes of cirrhosis, have additional factors that contribute to cardiac dysfunction, but cardiomyopathy can occur from any cause of cirrhosis.
HIGH YIELD POINT: When patients have cirrhosis, they have decreased SVR (due to the increased levels nitric oxide) and increased cardiac output. As a result, the EF will increase and can look falsely normal. Therefore, if a patient with cirrhosis has a reduced EF (<50%), this is highly concerning for HFrEF even though the EF may seem normal!!
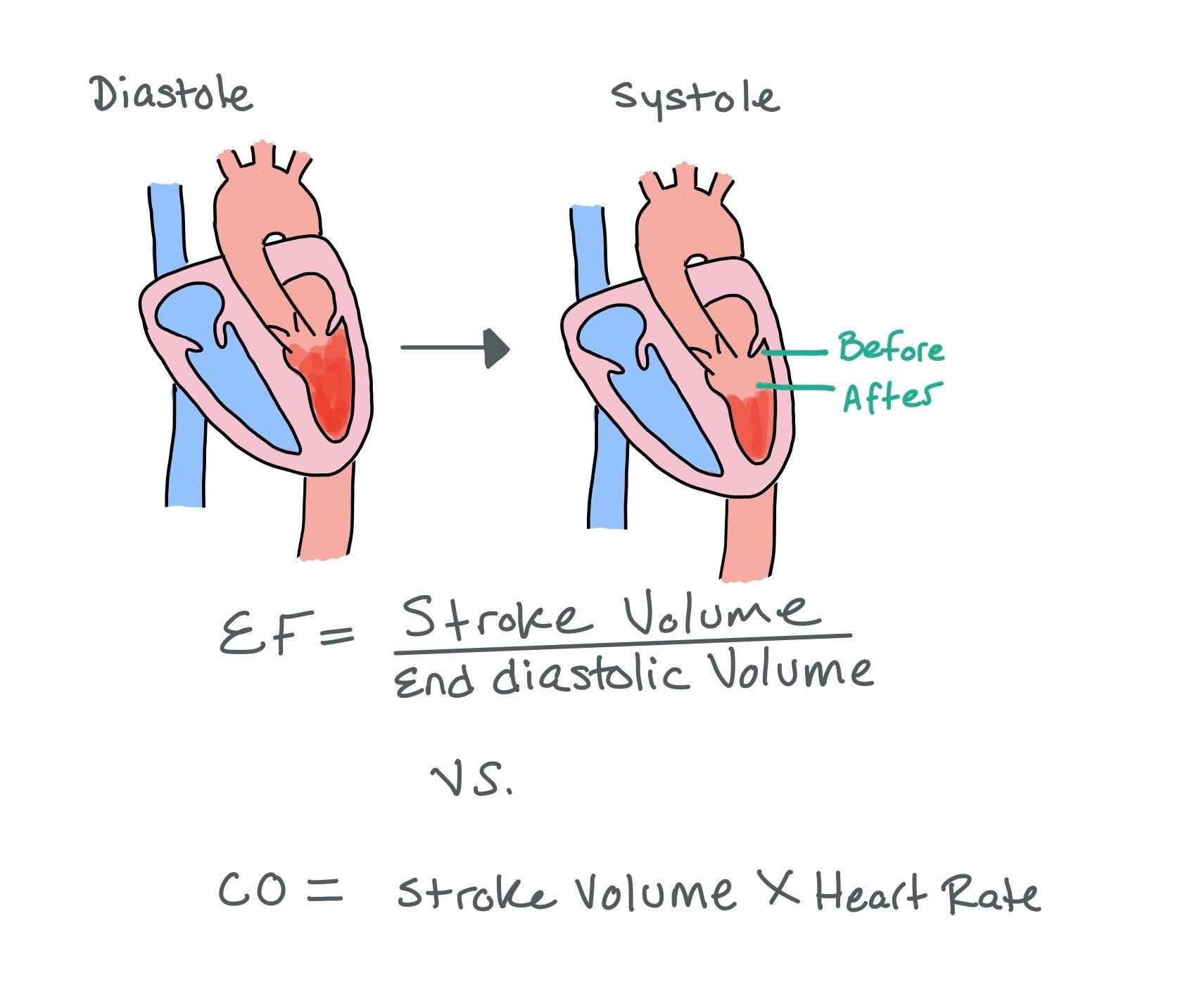
EF = SV / End Diastolic Volume
CO= SV x HR
Stroke volume will increase as SVR decreases, causing an apparent increase in both CO and EF.
The picture above shows how ejection fraction (EF) is calculated. Note how there is always blood left after systole and how the heart does not pump out all of the blood in the left ventricle. As a result, normal EF will always be ~55-60%, meaning the heart pumps out 55-60% of what was pumped in during diastole.
Hepatic Cardiomyopathy:
Hepatic cardiomyopathy: Cirrhosis causing cardiac disease:
Alcoholic cardiomyopathy is driven by oxidative damage caused by alcohol and its metabolites.
Iron overload from conditions like hemochromatosis accumulates in the epicardium through the endocardium, leading to membrane permeability alterations, resulting in myocyte death.
Thiamine deficiency, also considered wet beriberi, leads to cardiomyopathy as cellular metabolism impairment leads to vasomotor depression. This causes an increase in systemic vascular resistance which then translates to myocardial weakness with a low-output state.
High output heart failure: Cirrhotic cardiomyopathy is a clinical syndrome characterized by elevated cardiac output and contractility at rest but blunted stress response1. Patients will tend to present with elevated ejection fraction when at rest or in stable medical condition, presenting as heart failure with preserved ejection fraction (HFpEF)5.
Decompensated liver cirrhosis leads to a state of systemic peripheral vasodilation with low-resistance hyperdynamic circulation. The heart then must increase its pressure to be able to pump blood through an altered vascular system that now has low enough resistance and accumulates in the venous system rather than efficiently returning to the heart. Results of experimental studies indicate the involvement of several mechanisms in the pathophysiology, such as reduced beta-adrenergic receptor signal transduction, altered transmembrane currents and electromechanical coupling, nitric oxide overproduction, and cannabinoid receptor activation as contributing factors to this low-resistance hyperdynamic circulation.
The picture above depicts how cardiac dysfunction can lead to liver dysfunction and vis versa. Recognizing that dysfunction of one organ causes dysfunction of the other is imperative to thinking about progression of disease process.
Cardiac Cirrhosis:
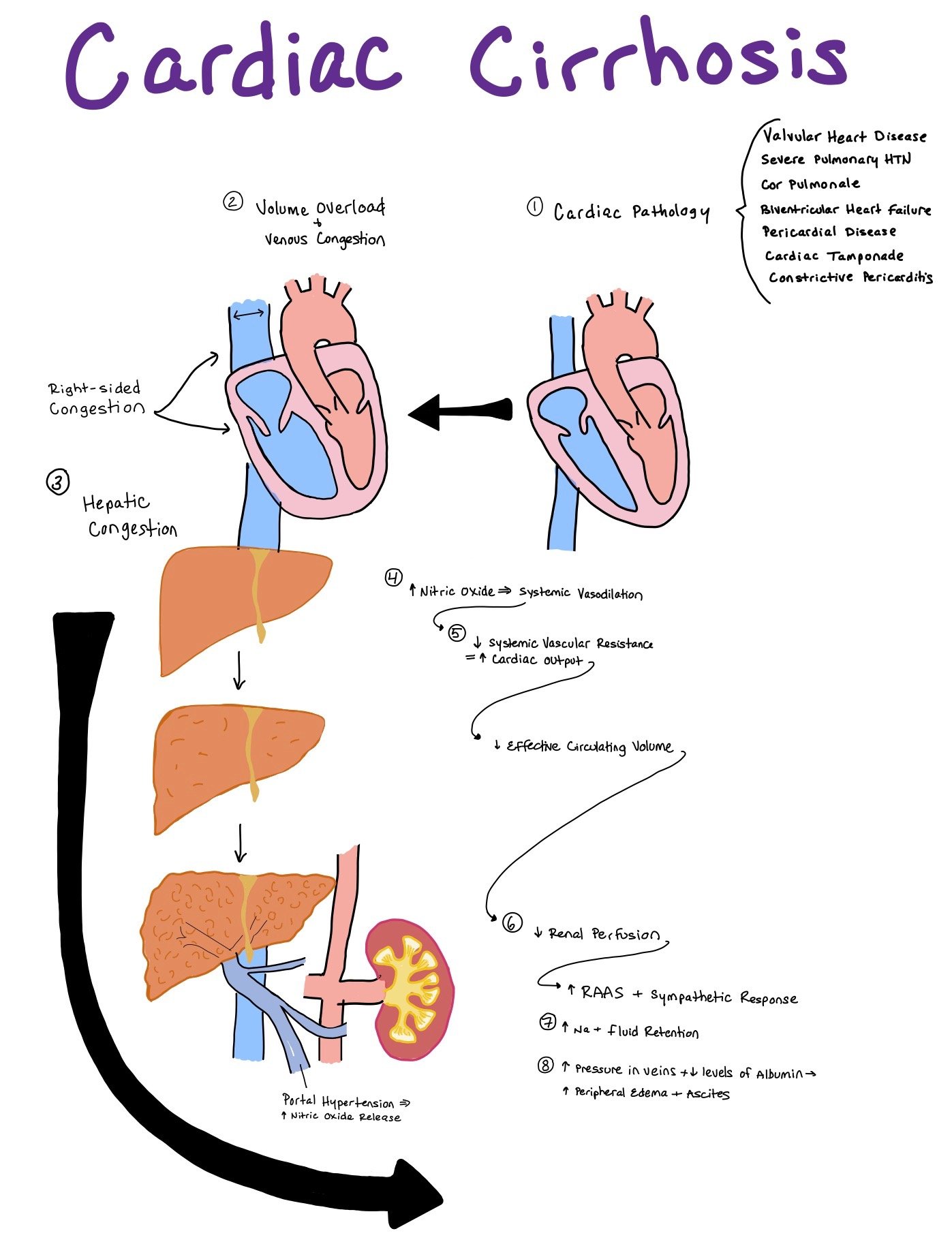
Cardiac Cirrhosis: Cardiac dysfunction can causes cirrhosis
In cardiac cirrhosis, the heart dysfunction occurs before the cirrhosis. Cardiac cirrhosis can occur due to
Valvular heart disease
Severe Pulmonary HTN
Cor Pulmonale
Biventricular Heart Failure
Pericardial Diseases
Cardiac Tamponade
Constrictive Pericarditis
The main reason hepatic dysfunction occurs is due to increased congestion from the heart into the hepatic veins and sinusoids that causes intrahepatic edema and decreased perfusion to the liver. Over time, this causes alteration of the hepatocyte architecture with atrophy, collagen deposition, and fibrosis to the hepatic veins and sinusoids.
In addition to increased congestion, decreased perfusion due to poor cardiac output can also cause liver injury. As 25% of the total cardiac output goes to the liver, any significant decrease in cardiac output can negatively affect the liver.
Reasons for Shortness of Breath:
When patients have both cardiac and liver dysfunction, sometimes it can be difficult to distinguish the cause of their shortness of breath. While dyspnea due to cardiac pathologies can be caused by pulmonary vascular congestion, dyspnea due to cirrhosis can be more complex and can be caused by
Hepatopulmonary syndrome
Portopulmonary HTN
Hepatic hydrothorax (less commonly)
1. Hepatopulmonary syndrome:
There is systemic vasodilation throughout the body due to increased nitric oxide formation. The body’s arterial system dilates to help counteract the poor blood flow. However, unlike all the other organs in the body, the lungs usually vasoconstrict when they don't get enough oxygen in order to help better oxygenate the parts of the lung that are perfused well. Unfortunately, due to dilation, the body is unable to do this. As a result, this creates a functional right to left shunt in which blood passes through the lung vasculature without being perfused. Perfusion > ventilation, shunting as no time for gas exchange.
Additionally in cirrhosis, the body creates multiple shunts from the portal to the arterial system to help offload the high pressures from the portal system. When these arteriovenous malformations are in the lungs, it can increase the A-a gradient and cause more dyspnea and hypoxemia.
On physical exam, patients will become more dyspneic when sitting up.
Dx:
TTE with contrast: When using agitated saline, you can see early bubbles in the left atrium (within 3-6 cardiac cycles) due to shunting.
CTPE can show distal vascular dilatation with subpleural telangiectasias, as well as slightly dilated subpleural vessels that extend to the pleural surface. Less commonly, arteriovenous malformations may be seen on peripheral pulmonary vessels
Nuclear Tc-99m Micro-Aggregated Albumin Scan: Allows for detection of intrapulmonary vascular dilatation by showing diffuse radionuclide uptake caused by right to left shunts.
Radiographically, this presents as distal vascular dilation with a large number of distal terminal vessel branches all concentrated in the lower lung bases.
2. Portopulmonary HTN:
Pulmonary artery hypertension caused by portal HTN (occurs in 2-5% of patients with cirrhosis). When this occurs, pulmonary artery pressure >25 mmHg (elevated) with PCWP <15 (normal).
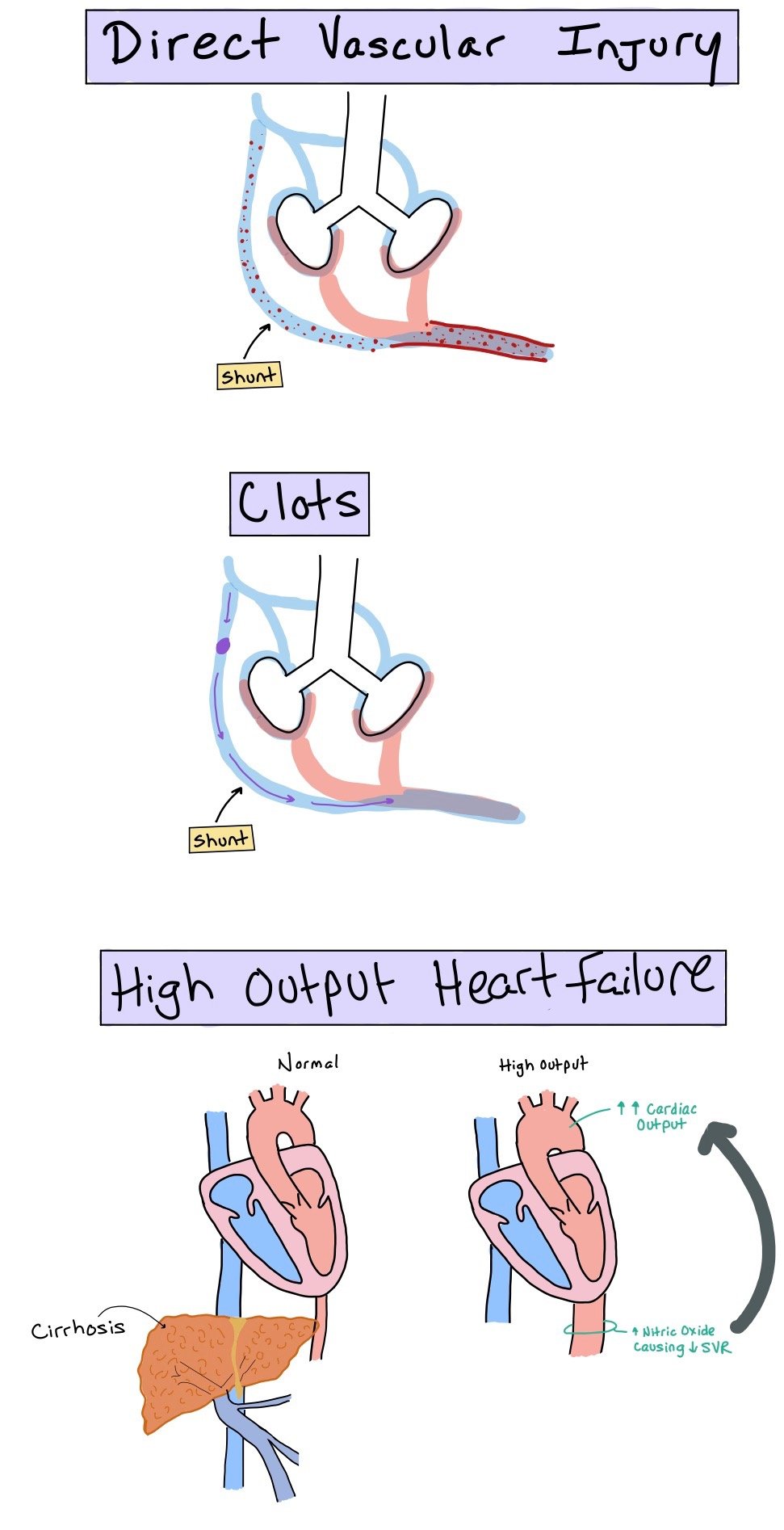
Direct injury to the vasculature
This occurs due to toxins that bypass the liver (via the portosystemic shunts) and directly injure the vasculature
Clots
Venous thromboembolisms that go through the portosystemic shunt into the pulmonary circulation
High output cardiac failure
As SVR decreases due to the increased nitric oxide, CO increases. When this happens, this causes increased stress to the pulmonary vascular bed. Overtime, this can cause proliferation of the pulmonary arterial endothelial cells.
Diagnosis:
Best diagnosed through catheterization, which will show elevated pulmonary pressures >25 mmHg with normal pulmonary capillary wedge pressure (PCWP) <15. This suggests pulmonary HTN rather than cardiac etiologies
3. Hepatic Hydrothorax:
Portal hypertension that causes >500mL of pleural effusion. As the peritoneal pressure increases within the abdomen, this can cause ascitic fluid to move into the pleural space through diaphragmatic defects. When this occurs, pleural fluid analysis will show serous transudate. Diagnosis can also be made via injecting Tc-99m sulfur colloid radiotracer into the peritoneal space and seeing if the radiotracer moves into the pleural cavity.
DX: CXR with a large, unilateral pleural effusion (picture on the right)
Putting it all together…
In order to treat these complex patients who have both heart and liver failure, it is important to pay close attention to the HPI, physical exam, labs, and imaging. Many times it is apparent from the history that the patient had underlying cirrhosis that may have precipitated heart failure or vice versa. In that case, treating the underlying disorder (i.e. preventing further drinking and hepatic dysfunction) can help prevent worsening dysfunction of the other organ (in this case, the heart).
If these patients present with dyspnea, it can be difficult to decipher what is actually causing the shortness of breath. If patients have elevated BNP, JVD, pulmonary vascular congestion, and shortness of breath that worsens with laying flat, this may point towards fluid overload secondary to acute decompensated heart failure. On the other hand, if the BNP is normal and there is no JVD or pulmonary vascular congestion, this may suggest the dyspnea is due to the cirrhosis. In this case, hepatopulmonary syndrome, portopulmonary HTN, and hepatic hydrothorax should be ruled out.
If it is still difficult to determine, right heart catheterization can also be used to help give more definitive answers to the patient’s hemodynamics.
Regardless, a multidisciplinary approach to treatment with both cardiology, hepatology, and pulmonology is ideal.
Back to the Case:
1. What could be contributing to new edema and distention?
Heart failure exacerbated by new hepatic decompensation with ascites and altered liver function tests. Heart failure with preserved, or hyperdynamic LV function, is common in cirrhosis, presenting as diastolic dysfunction. Due to increased atrial pressures and limited filling capacity, volume gets backloaded is then third-spaced into abdomen (ascites), lower extremities (edema) and lungs (pulmonary vascular congestion).
2. What labs, tests, and images should you order?
BNP, troponin, CBC to rule out infection and anemia, LFTs, and infectious workup, including paracentesis if the patient has ascites to rule out spontaneous bacterial peritonitis (SBP).
You can also order a TTE if you are concerned for new or worsening HF. RUQ US can be ordered to examine liver cirrhosis damage/scarring with possible follow-up MRI abdomen
3. How might the patient having a fever complicate his presentation?
If the patient is febrile, his decompensation may be driven by sepsis. In sepsis, there is systemic vasodilation, which causes decreased SVR and an increased CO. As patients with both heart failure and cirrhosis already have complex hemodynamics, the addition of sepsis can overload the system and cause severe decompensation.
4. What do you think about his bedside POCUS?
When patient’s have cirrhosis, we expect higher cardiac outputs and EFs due to the decreased SVR. This patient has a lower EF of 45%, suggesting HFrEF. As such, while this patient has known cirrhosis, we have to have a high index of suspicion for concomitant secondary heart failure. We must recognize that a lower EF in patients like this is not normal as their EFs are usually falsely elevated due to the decreased SVR and resultant elevated stroke volume.
5. How can we best improve the shortness of breath?
Try to use history, physical exam, labs, and imaging to help determine what is causing the shortness of breath. If the patient is in decompensated heart failure, diuresis may be the best option. However, if the patient has decompensated cirrhosis, strategies aimed at improving the cirrhosis (such as octreotide, midodrine, etc) may be beneficial.
In this case, the patient has acute decompensated heart failure, seen by his elevated BNP, JVD, and pulmonary vascular congestion, so he would benefit from diuresis.
Further Learning:
Resident Responsibilities:
○ Confirm no other infection is leading the exacerbation. Perform paracentesis to rule-out SBP, check Chest xray for effusions/pneumonia, check labs to confirm no leukocytosis, adequate electrolyte management given underlying heart failure diagnosis, renal function to rule out hepatorenal syndrome.
○ Consult Hepatology if not on service, and consult Cardiology for heart failure, consider consulting nephrology for suspected HRS vs AKI
○ Given cardiomegaly and peripheral edema, obtain TTE to evaluate heart function
○ Obtain thorough patient history for recent NSAID, acetaminophen, other drug use, recent decompensations requiring hospitalization
○ Monitor UOP to ensure appropriate diuresis and monitor renal function to ensure improvement
○ Monitor chest x-ray to observe vascular congestion and monitor oxygen requirement changes, ensuring pleural effusions don’t occur.
Further Readings:
Hepatopulmonary Syndrome:
https://radiopaedia.org/articles/hepatopulmonary-syndrome?lang=us
Portopulmonary HTN:
https://radiopaedia.org/articles/portopulmonary-hypertension?lang=us
Hepatic Hydrothorax:
https://radiopaedia.org/articles/hepatic-hydrothorax?lang=us
How’d we do?
The following individuals contributed to this topic: Laura Fernandez Morales, MD, Sean Tomkins, MD, PhD
Chapter Resources
Zardi EM, Abbate A, Zardi DM, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56(7):539-549. doi:10.1016/j.jacc.2009.12.075
Shaaban A, Gangwani MK, Pendela VS, Vindhyal MR. Alcoholic Cardiomyopathy. In: StatPearls. StatPearls Publishing; 2023. Accessed August 1, 2023. http://www.ncbi.nlm.nih.gov/books/NBK513322/
Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron Overload Cardiomyopathy, Better Understanding of An Increasing Disorder. J Am Coll Cardiol. 2010;56(13):1001-1012. doi:10.1016/j.jacc.2010.03.083
Helali J, Park S, Ziaeian B, Han JK, Lankarani-Fard A. Thiamine and Heart Failure. Mayo Clin Proc Innov Qual Outcomes. 2019;3(2):221-225. doi:10.1016/j.mayocpiqo.2019.03.003
Milani A, Zaccaria R, Bombardieri G, Gasbarrini A, Pola P. Cirrhotic cardiomyopathy. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2007;39(6):507-515. doi:10.1016/j.dld.2006.12.014
Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53(1):179-190. doi:10.1016/j.jhep.2010.02.023
Zardi EM, Zardi DM, Chin D, Sonnino C, Dobrina A, Abbate A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J Cardiol. 2016;67(2):125-130. doi:10.1016/j.jjcc.2015.04.016
Timoh T, Protano MA, Wagman G, Bloom M, Vittorio TJ. A perspective on cirrhotic cardiomyopathy. Transplant Proc. 2011;43(5):1649-1653. doi:10.1016/j.transproceed.2011.01.188
Danielsen KV, Wiese S, Busk T, et al. Cardiovascular Mapping in Cirrhosis From the Compensated Stage to Hepatorenal Syndrome: A Magnetic Resonance Study. Off J Am Coll Gastroenterol ACG. 2022;117(8):1269. doi:10.14309/ajg.0000000000001847