Case Presentation:
A 65 year old male with past medical history of HTN and HLD presents to the ED with a 6 month history of progressive dyspnea on exertion and lower leg swelling. He states that he used to walk and run marathons with no issues, but now he cannot walk more than a few blocks before getting short of breath. Vitals are notable for T 98o, BP 160/98, HR 103, and 98% on 2L. Physical exam is notable for JVD, 2+ pitting edema, and bibasilar crackles in the lungs. You quickly do a bedside TTE while the patient is still in the ED, and you notice that there is diffuse thickening of throughout the heart but that the patients EF is grossly normal.
Ask Yourself:
Questions:
1. How would you stage and classify this patient’s heart failure and symptoms?
2. What tests do you want to get during your workup for this patient?
3. What treatment options are available for this patient?
Background:
Figure 2 shows the Forrester Classification of different classes of acute decompensated heart failure based on how well they are perfusing and how congested they are. The different classes, including warm and dry (the best category as it depicts volume overload but good perfusion) to cold and wet (worst because it means volume overload with poor perfusion) and how to treat them (with diuretics vs inotropic agents). When patients are more congested, they will have symptoms of increased fluid overload, such as shortness of breath, lower extremity edema, paroxysmal nocturnal dyspnea, and early satiety. When patients have decreased perfusion, they may be more confused, have decreased urine output, increased lethargy, and may feel lightheaded and nauseous.
This patient is presenting with signs concerning for new onset heart failure (HF). HF is a disorder in which the heart is not able to pump blood effectively to the rest of the body. This can be due to structural issues or problems with the function of the heart, specifically with filling (i.e. diastole) or squeezing of the heart (systole). The diagnosis of HF is a clinical diagnosis. We use history, clinical signs and symptoms, laboratory work up, and imaging to help solidify this diagnosis.
When patients present in acute decompensated heart failure, it’s important to be able to classify their heart failure, specifically in terms of how well they are perfusing (i.e. how good their cardiac output is) and how congested they are (i.e. how much fluid they are retaining). Figure 2 demonstrates the Forrester Classification for heart failure and how we can treat patients based on their presentation and symptoms.
Anytime someone is coming in with concerts for new heart failure, it is important we get the following labs and tests in order to help us understand why they are now in heart failure:
Baseline labs: Troponin, BNP, lactate, CBC, BMP, TSH, lipid panel, A1c, urine hCG, iron studies, HIV testing, and LFTS s
CXR: To see increased pulmonary vascular congestion vs pneumonia
TTE: To evaluate the ejection fraction of the left ventricle and to look for any signs of systolic/diastolic dysfunction or wall motion abnormalities. The TTE is important because it gives us a good estimate of the LV ejection fraction (EF). The EF is important in differentiating HFrEF from HFpEF. The diagnosis of HFrEF is made with a left ventricular EF <40%.
EKG/Telemetry: Many times, prolonged tachycardia can actually produce a tachy-induced cardiomyopathy and cause heart failure. Therefore, ruling out underlying arrhythmias are crucial.
Catheterization: In many cases, we need to be able to determine if ischemia is the cause of heart failure as underlying CAD, HTN, and DM are major leading causes of HF.
Remember! It is also important to rule out high output etiologies, such as cirrhosis, anemia, hyperthyroidism, Pagets disease, and AV shunt, for reasons of the heart failure.
Staging vs. Functional Status
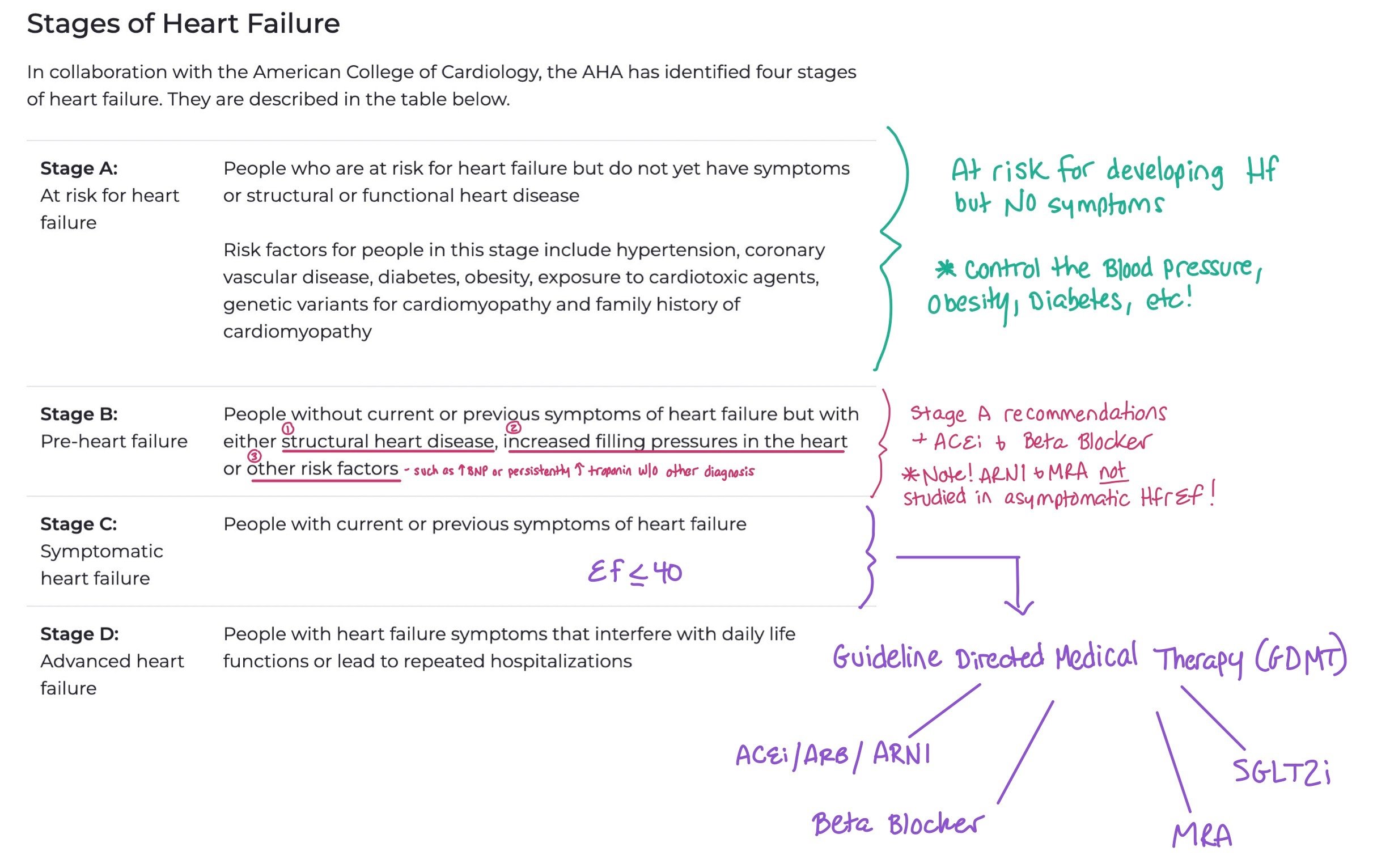
When we talk about heart failure, we typically refer to both the stage of the heart failure and the functional status, i.e. the New York Heart Association (NYHA) Functional Classification, the patient has due to the heart failure. Knowing the staging allows providers to understand how advanced the heart failure is, while providing the functional classification allows them to assess and treat patient symptoms. Staging refers to progression of heart failure, while function refers to symptoms.
This picture breaks down the different stages of heart failure. When patients have Stage A, the main intervention is trying to control their risk factors for developing heart failure, such as controlling their blood pressure, diabetes, and obesity. For Stage B, you can also add an ACEi and Beta Blockers. Finally, for Stage C and D with HFrEF < 40, this is when we start to add the GDMT.
Note: Unlike functional status that is constantly changing, stage progression only goes in one direction (i.e. once patients have Stage C they cannot go back to stage B).
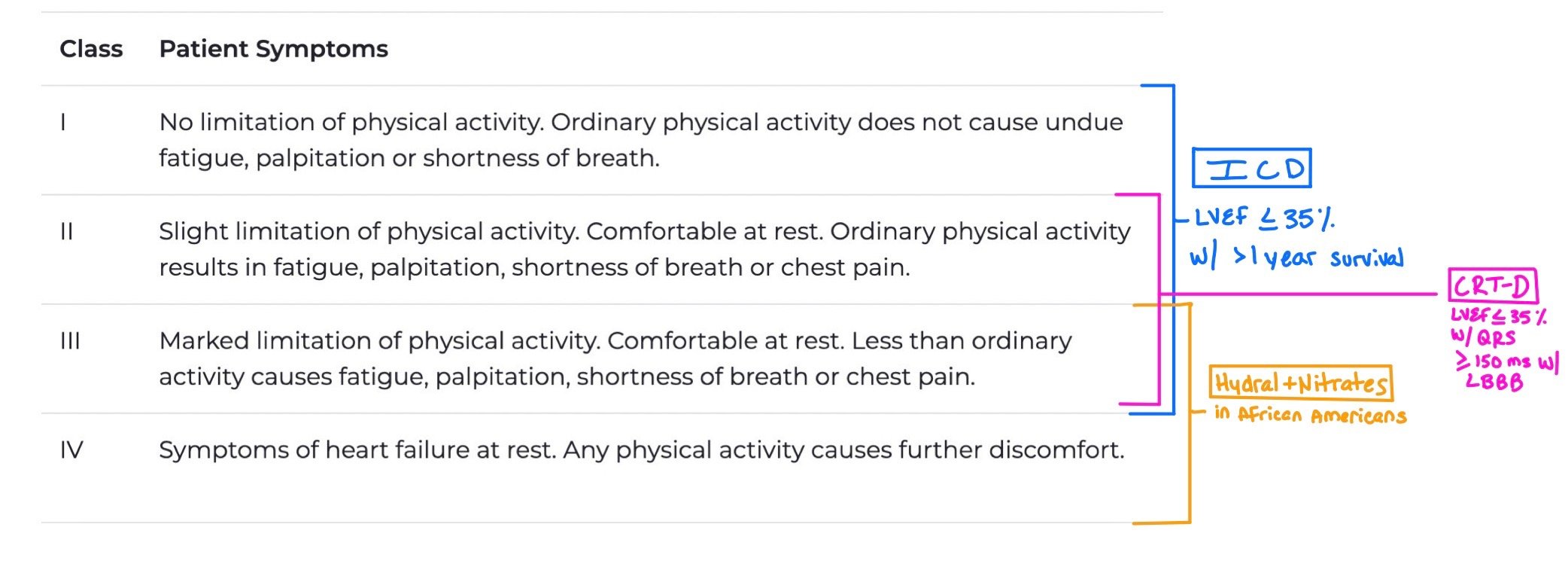
The NYHA Functional Classification is based on the patient’s symptoms, which can fluctuate based on whether or not the patient is decompensated or not.
When patients have Class I-III with LVEF < 35% and greater than 1 year survival, they should get an ICD.
When patients have Class II-III with LVEF < 35% with QRS > 150 ms and left bundle branch block (LBBB), they are candidates for CRT-D.
Patients with Class III-IV who are African American should get hydralazine + nitrate
The following charts from the 2022 AHA/ACC/HFSA Heart Failure Guidelines help to demonstrate how we use both staging and functional class to treat our patients. The first chart shows how we go about treating patients with Stage A and B, while the second picture shows treatment for Stage C and D.
This figure shows treatment for Stages A and B. The green boxes denote Class 1 recommendations (strong evidence with high benefits) while yellow is Class 2a (moderate evidence). As patients in Stage A are classified at risk for developing heart failure, therapies are targeted to help them prevent progression by treating risk factors. For Stage B, we continue to try to prevent progression by treating underlying disease; however, when patients start to develop HFrEF (i.e. EF <40) these patients benefit from ACEi and beta blockers. If they qualify for an ICD due to EF < 30% >40 days after MI, this is considered a Class 1 recommendation.
This figure shows how we treat our patients with Stages C and D. Remember, these patients have already become symptomatic from their heart failure. Therapies are targeted to help prevent further disease progression with guideline directed medical therapy (GDMT) as well as additional therapies depending on their NYHA functional class if they continue to be symptomatic despite the fact that they are on maximally tolerated GDMT.
What about our patients with HFpEF?
Until recently, we did not have good therapies to treat patients who have diastolic dysfunction, aka heart failure with preserved ejection fraction (HFpEF, EF >50%). As ~50% of our patients with heart failure have HFpEF, determining therapies for these patients became critical. While the mainstay of therapies is to treat underlying risk factors (like hypertension, diabetes, renal disease, etc), we try to help these patients with symptom management, specifically with diuretics. SGLT2i are given a Class 2a recommendation as they help to augment diuresis and have been shown to decrease hospitalizations and length of stay (EMPEROR-PRESERVE),
The following figure from the 2022 AHA/ACC/HFSA Heart Failure Guidelines demonstrates HFpEF treatment. Note how ARNI, MRA, and ARBs are class 2b (i.e. weak recommendations). While not first line, they can be used to help control HTN and potential further progression of the patient’s heart failure.
Cardiac Amyloidosis:
One major cause of heart failure, especially HFpEF, is Cardiac Amyloidosis.
Amyloid is a generic term for misfolded proteins that form beta pleated sheets. Since the body is unable to breakdown these proteins, the proteins will deposit into different parts of the body. Cardiac Amyloidosis, the most common type of restrictive cardiomyopathy, occurs when the amyloid deposits within the myocardium.
While there are many different types of proteins that can do this, we divide those proteins into two main groups:
AL Amyloidosis– This is a plasma cell disorder in which plasma cells make antibodies, which have body heavy and light chains. The light chains dissociate and come together to make the misfolded protein of amyloid. This is a toxic infiltrative disease as the amyloid deposits and causes myocyte apoptosis.
ATTR– Occurs when the transthyretin protein, a protein made in the liver involved in the transportation of thyroxine and retinol, misfolds. There are two forms of this subtype:
TTRm — The mutant / familial form in which there is a problem with the proteins themselves.
TTRwt — The wild-type (formally known as the senile form) etiology occurs when the protein itself is normal but gets misfolded
The reason it’s important to understand the different types of amyloid is because the workup and treatment are very different between the two.
In this picture, you can see how both the TTR and AL amyloid are created and deposit in the heart. The TTR tetramer protein is created in the liver. The tetramer dissociates, and then one of the proteins gather to create beta pleated sheets that the body cannot break down. In the AL version, the bone creates plasma cells that then create immunoglobulin. The light chains from the immunoglobulin come together to also form beta pleated sheets and deposit in the heart.
When to Be Suspicious of Amyloid:
Signs and Symptoms: Patients usually present with signs of RV failure, such as elevated jugular venous pressure, hepatomegaly, ascites, and peripheral edema. Additionally, you should ask about neuropathic issues as well, such as bilateral carpal tunnel, peripheral neuropathy, autonomic dysfunction (like orthostatic hypotension), and spontaneous bicep tendon rupture (i.e. Popeye’s sign). These can also occur due to amyloid deposit on the nerves. Some patients may even have macroglossia and periorbital purpura.
Labs:
AL – Make sure to get serum free light chain assay and both serum and urine immunofixation. Note: This is NOT SPEP and UPEP, which are ordered in order to diagnose multiple myeloma.
TTR- Unfortunately, there are no tests for diagnosis. However, if patients are diagnosed with TTR amyloid, they will need genetic tests.
Patients may also have nephrotic range proteinuria!
EKG: You MAY see low voltage in ~60% of cases in AL and 20% of TTR, so it isn't really sensitive or specific. However, it is very common to see a disproportionate voltage between the EKG vs how much tissue / muscle the patient has seen on TTE. You may also see pseudoinfarct patterns with Q waves in the anterior and inferior leads when patients do not have any current or past obstruction, You may also see arrhythmias, like afib, or signs of conduction disease, such as heart block.
TTE:
Common signs are diffuse thickening in throughout the heart, especially in the intra-atrial septum, the ventricles, and even in the RV free wall, The thickened myocardium may even have a sparkling appearance (Figure 4)
Diastolic dysfunction
Low tissue velocities
Abnormal strain despite normal EF
Apical sparing on strain imaging (i.e. “bull’s eye)
Pericardial Effusions
These pictures show both a normal heart (top) and heart with amyloid (bottom) as seen via parasternal long echocardiogram. Notice how the picture of the amyloid heart has very thick walls.
A TTR Amyloid Scoring system by Davies et al. also exists which can be used to help risk stratify patients with good sensitivity and specificity. Patients receive scores based on:
Age >60
Male sex
HTN
Relative wall thickness more than 0.57
Posterior wall thickness of > 12 mm
EF <60%
Further Workup:
Bone Scintigraphy: AKA the Tc-99m PYP Scan. In this study, radiolabeled pyrophosphate (PYP) is injected in the body. Usually, this tracer is only taken up in the bones. However, if patients have TTR amyloid, you may also see tracer uptake in the heart. Note: This test is NOT used for the AT subtype!!
Cardiac MRI: Many times, we can see late gadolinium enhancement (either subendocardial or global transmural). Unfortunately, this modality does not let us distinguish between the AL vs TTR subtype. Plus, these can be difficult to perform as many times patients with amyloidosis have afib, which makes the study technically difficult.
Biopsy:
AL: You can biopsy a fat pad or the bone marrow (as plasma cells are made in the bones)
TTR: Biopsy needs to be in the heart
Ultimately, we take these biopsies and stain them with congo red. We then send them for mass spectrometry to help evaluate for the specific protein causing the amyloidosis.
In Figure 5, we see a Tc-99m PYP Scan. These are graded from 0-3. Notice how Grade 0 only has radiotracer in the bones, while Grades 2 and 3 have uptake in the heart as well. The more tracer there is in the heart, the more likely it is the patient has amyloid. Again, remember that this is ONLY for diagnosing TTR amyloid!!
Figure has been adapted. Original figure courtesy of Masri A, et al. Circulation. 2020;13:e010249
Treatment:
Prompt diagnosis and early treatment are paramount, as it prevents disease progression.
Heart failure: When we can, treating with guideline directed medical therapy (GDMT) is important and should be done as tolerated. Unfortunately, these patients tend to not tolerate GDMT as well as typical heart failure. Therefore, we need to add on agents carefully. Diuretics are very important as they help us maintain euvolemia and control symptoms.
Atrial Fibrillation: Anyone with confirmed amyloid who has afib MUST be anticoagulated, regardless of CHA2DS2-VASc! It is also important to ensure we control the rate or rhythm as prolonged prolonged tachycardia can cause worsening cardiomyopathy!
Devices: There is conflicting data about whether or not patients with amyloid should get devices. However, they tend to get them when they have conduction disease (like heart blocks) and ventricular arrhythmias to prevent sudden cardiac death
Targeted AL Therapies
- Will be treated with chemotherapy for plasma cell disorders with the help of hematology
Targeted TTR Therapies
- Tefamidis, a recently FDA approved drug, helps to stabilize the original tetramer so that it does not break apart and allow those individual parts form together to cause amyloidosis and deposit in the tissue.
- TTR silencers, such as Patisiran and Inotersen, help prevent translation of the mRNA into protein.
- CRISPR Gene Editing - Allows for us to turn off the TTR gene
Pathway of Amyloid:
Back to the Case:
1. How would you stage and classify this patient’s heart failure and symptoms?
Given the fact that this patient has current symptoms of heart failure, he would be classified as Stage C (i.e. symptomatic heart failure). As his symptoms are not limiting his daily life functions and have not lead to repeated hospitalizations, he does not yet have Stage D or advanced heart failure. As for his NYHA class, this patient would be Class II-III as he is comfortable at rest but becomes more short of breath and fatigued with physical activity.
2. What tests do you want to get during your workup for this patient?
All patients with heart failure should get baseline lab workup (i.e. troponin, BNP, lactate, CBC, BMP, lipid, A1c, LFTs, HIV etc), EKG, and TTE. If patients have newly reduced EF, they may also be candidates for catheterization to rule out ischemic causes of their reduced EF. This patient, however, needs to be worked up for amyloid as he has new HFpEF in his 60s. Initial workup includes getting a serum free light chain and both serum and urine immunofixation to rule out AL amyloid. If these tests come back negative, TTR amyloid needs to be ruled out with a PYP Scan (i.e. bone scintigraphy) or cardiac MRI. In both AL and TTR, biopsy can be used to clinch the diagnosis.
3. What treatment options are available for this patient?
AL amyloid is treated with plasma cell directed therapies with the help of hematology, while TTR amyloid can be treated with Tefamidis, TTR silencers, and CRISPR gene editing.
**Regardless of whether or not the patient has AL or TTR amyloidosis, he should be put on GDMT with extra emphasis on diuretics to help maintain euvolemia and control symptoms. Remember, patients with amylod tend to not tolerate GDMT as well as other patient with heart failure, so these medications should be added on with caution.
Further Learning:
Resident Responsibilities
Any patient coming in with new heart failure should get roponin, BNP, CBC, BMP, TSH, lipid panel, A1c, urine hCG, iron studies, HIV testing, and LFTS s
Make sure to take a good history, especially focusing on neuropathic symptoms, such as carpal tunnel and neuropathies
Recognize the difference in diagnosis and treatment of AL vs TTR. If patients have the AL subtype, it is important to get Hematology on board early!
Remember that patients with amyloid might not tolerate GDMT as well as our typical HF patients. As such, add them on slowly!
Any patient with amyloid and AF MUST be on anticoagulation regardless of CHA2DS2-VASc!
Further Learning:
The Curbsiders Internal Medicine Podcase: #427: Kittleson Rules Amyloidosis
ATTR-ACT Trial- A landmark trial that showed how effective tafamidis was at helping reduce mortality in patients with TTR amyloid.
“Cardiac Amyloidosis: Update on Diagnosis and Treatment” by Dr. Kunal Bhatt, Emory Cardiology
https://www.youtube.com/watch?v=WbUVa_4ancA
2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for Patients with Cardiac Amyloidosis
https://www.jacc.org/doi/10.1016/j.jacc.2022.11.022
A Simple Score to Identify Increased Risk of Transthyretin Amyloid Cardiomyopathy in Heart Failure With Preserved Ejection Fraction. JAMA
Attending Pearls:
Make sure to get a serum free light chain assay and both serum and urine immunofixation on all patients with concerns of amyloid!
Look at the TTE yourself. If patients have amyloid, sometimes you can see protein deposition on the TTE, which can appear as a thickened myocardium with a sparkling appearance.
If the amplitude of the QRS is lower than expected given the thick myocardium, be suspicious of amyloid!
Be concerned for amyloid when patients are elderly and have HF with preserved EF or low flow. low gradient aortic stenosis. Also, new hypertrophic cardiomyopathy in patients > 60 years old is VERY concerning!!
Remember to get daily weights for all patients admitted for HF!
How’d we do?
The following individuals contributed to this topic: Romani Wahba, MD, Mary Rodriguez Ziccardi, MD
Chapter Resources
Connors, Lawreen H., et al. “Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study.” Circulation (New York, N.Y.), vol. 133, no. 3, 2016, pp. 282–90, https://doi.org/10.1161/CIRCULATIONAHA.115.018852.
Kittleson, M, Ruberg, F. et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023 Mar, 81 (11) 1076–1126. https://doi.org/10.1016/j.jacc.2022.11.022
Masri, Ahmad, et al. “Efficient 1-Hour Technetium-99 m Pyrophosphate Imaging Protocol for the Diagnosis of Transthyretin Cardiac Amyloidosis.” Circulation. Cardiovascular Imaging, vol. 13, no. 2, 2020, pp. e010249–e010249, https://doi.org/10.1161/CIRCIMAGING.119.010249.
Picken, Maria M., et al., editors. Amyloid and Related Disorders Surgical Pathology and Clinical Correlations. 2nd ed. 2015., Springer International Publishing, 2015, https://doi.org/10.1007/978-3-319-19294-9.
Treibel, Thomas A., et al. “Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and Prognosis in Patients Undergoing Surgical Aortic Valve Replacement.” Circulation. Cardiovascular Imaging, vol. 9, no. 8, 2016, https://doi.org/10.1161/CIRCIMAGING.116.005066.
Kristen, Arnt V., et al. “Cardiac Amyloid Load: A Prognostic and Predictive Biomarker in Patients With Light-Chain Amyloidosis.” Journal of the American College of Cardiology, vol. 68, no. 1, 2016, pp. 13–24, https://doi.org/10.1016/j.jacc.2016.04.035.
Cuddy, Sarah A. M., et al. “Improved Quantification of Cardiac Amyloid Burden in Systemic Light Chain Amyloidosis.” JACC. Cardiovascular Imaging, vol. 13, no. 6, 2020, pp. 1325–36, https://doi.org/10.1016/j.jcmg.2020.02.025.
Davies DR, Redfield MM, Scott CG, et al. A Simple Score to Identify Increased Risk of Transthyretin Amyloid Cardiomyopathy in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2022;7(10):1036–1044. doi:10.1001/jamacardio.2022.1781