Case Presentation:
A 35-year-old female with an unknown past medical history presents to the ED following a syncopal event. She has noticed occasional episodes of palpitations with activity, as well as fatigue and lightheadedness over the last three months. On further questioning, she mentions a history of heart surgery when she was a child; however, she does not know specifics and has not seen a physician in 20 years.
Vitals: BP: 150/92, HR: 100s, RR: 16, O2 96% on RA, T 99.0 F.
Labs: CBC and CMP within normal limits; troponin negative; NT-proBNP of 200 pg/mL
Physical exam:
Cardiac: Loud S2P with a systolic ejection murmur and loud early diastolic murmur at the left upper sternal border. Elevated JVP
Pulm: lungs clear to auscultation bilaterally
MSK: 2 + pitting edema bilaterally
Midline sternotomy scar present
Imaging:
CXR: Unremarkable
TTE: Notable for severe pulmonic regurgitation, moderate tricuspid regurgitation, right ventricular hypertrophy with increased RV end-diastolic volume, and a small ventricular septal defect. Normal LV size and function. Aortic root dilatation to 4.2cm
EKG Interpretation: Notable atrial fibrillation with rates in the 100s. Normal axis and intervals with incomplete right bundle branch block. No ST elevations / depressions or T wave inversions.
Ask Yourself:
What further workup is needed to determine this patient's diagnosis and next steps in management?
What long-term complications can arise in adult patients with surgically corrected hearts?
What sort of preventative healthcare could have improved this patient's presentation?
Background:
Epidemiology:
In the 1950s, 90% of infants born with complex cardiovascular diseases died in childhood.
Thanks to advancements in diagnostic studies, cardiac surgery, and intensive care, 85-90% of babies born with cardiovascular anomalies can reach adulthood in the United States
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, accounting for 7-10% of congenital heart disease cases
Associated with various genetic syndromes, including trisomy 21 and DiGeorge syndrome
Anatomy and Pathophysiology:
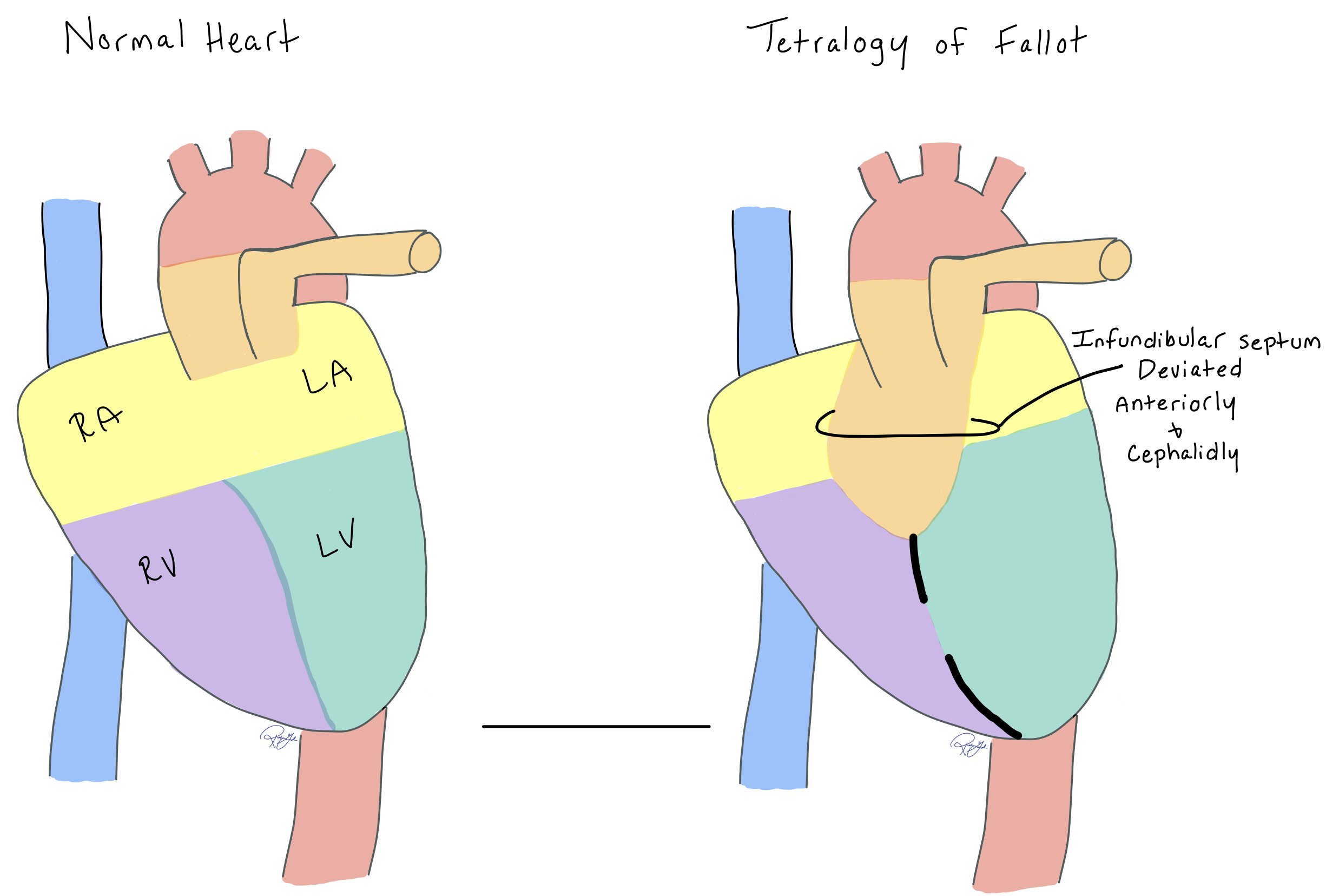
While the exact embryonic abnormality in TOF is unknown, anterior and cephalad deviation of the infundibular septum is one of the defining features of the disease.
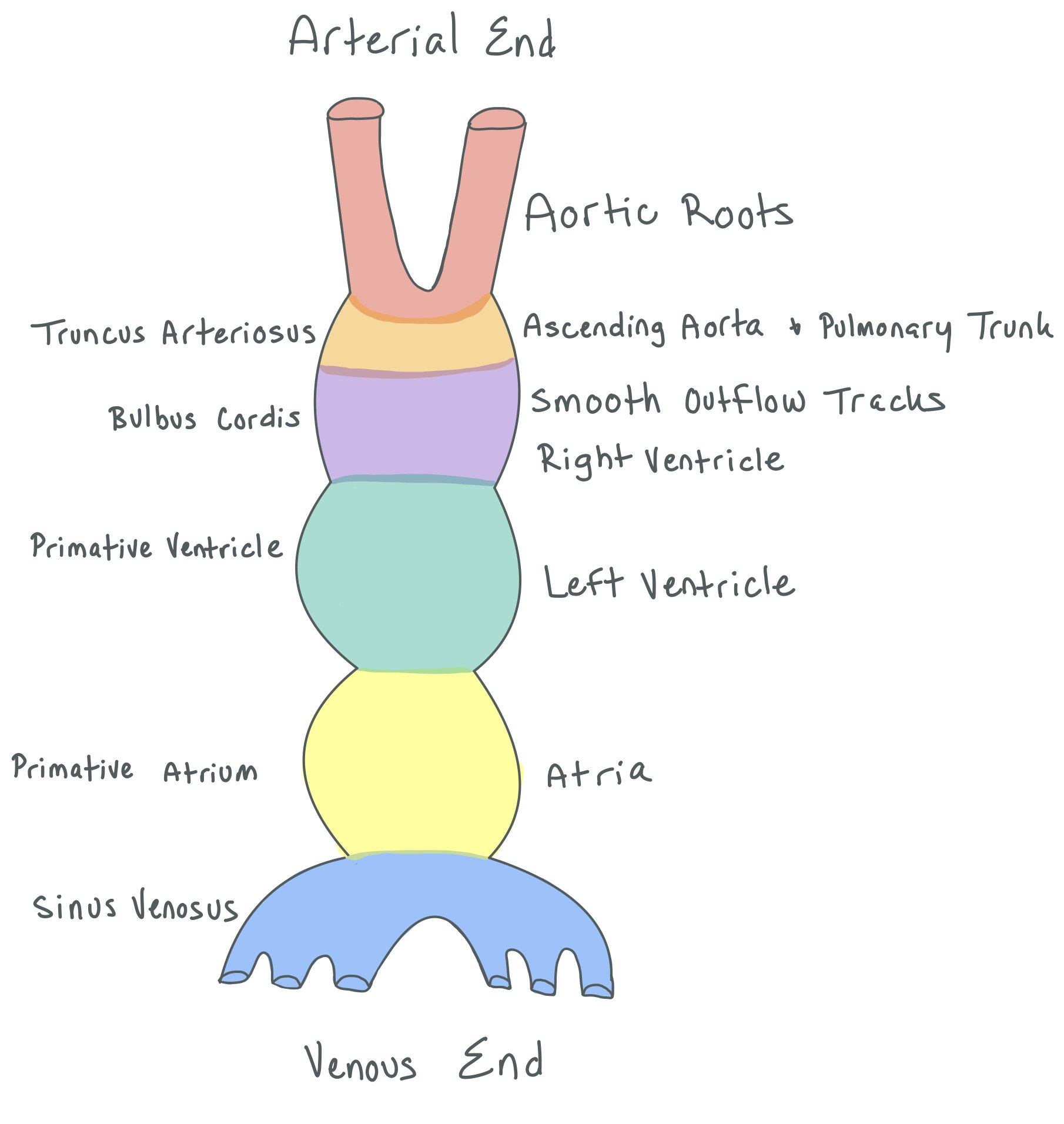
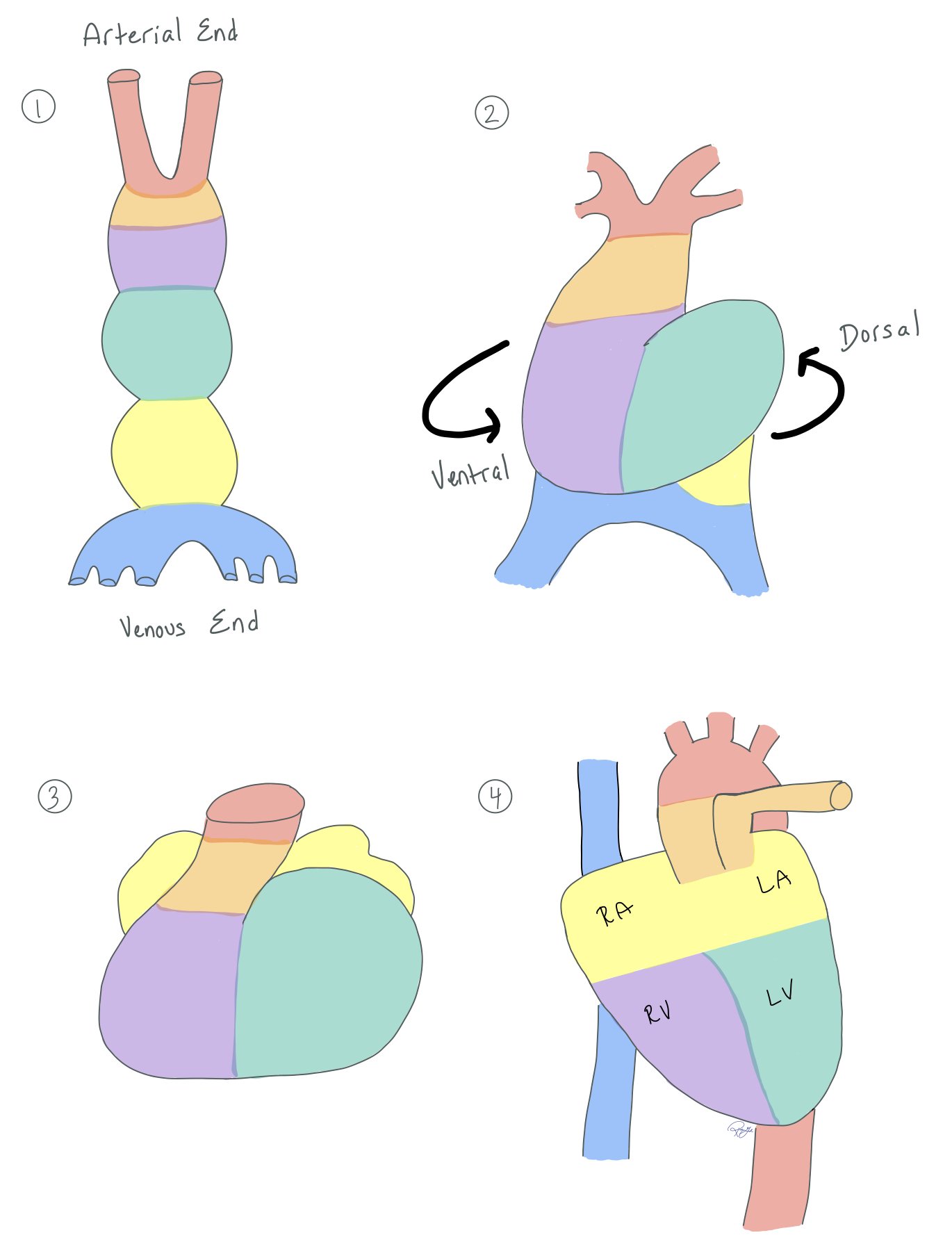
The figure above shows the embryonic development of the heart, which typically forms around day 22 of development.
In normal heart development, two tubes come together to make one big tube (picture 1). On day 23 of development, the heart tube begins to twist both dorsally and ventrally (picture 2).
The tube continues to twist (picture 3) until the heart has both a right and left atria and ventricles (picture 4). You can see how much the tube twists by noting how the colors shift from the top left picture 1 to the bottom right picture 4.
In TOF, there is abnormal anterior and cephalid deviation of the infundibular septum. This abnormality in development is the defining feature in the resulting anatomy.
Notice how the infundibular septum is deviated anteriorly and cephalidly in the heart with TOF.
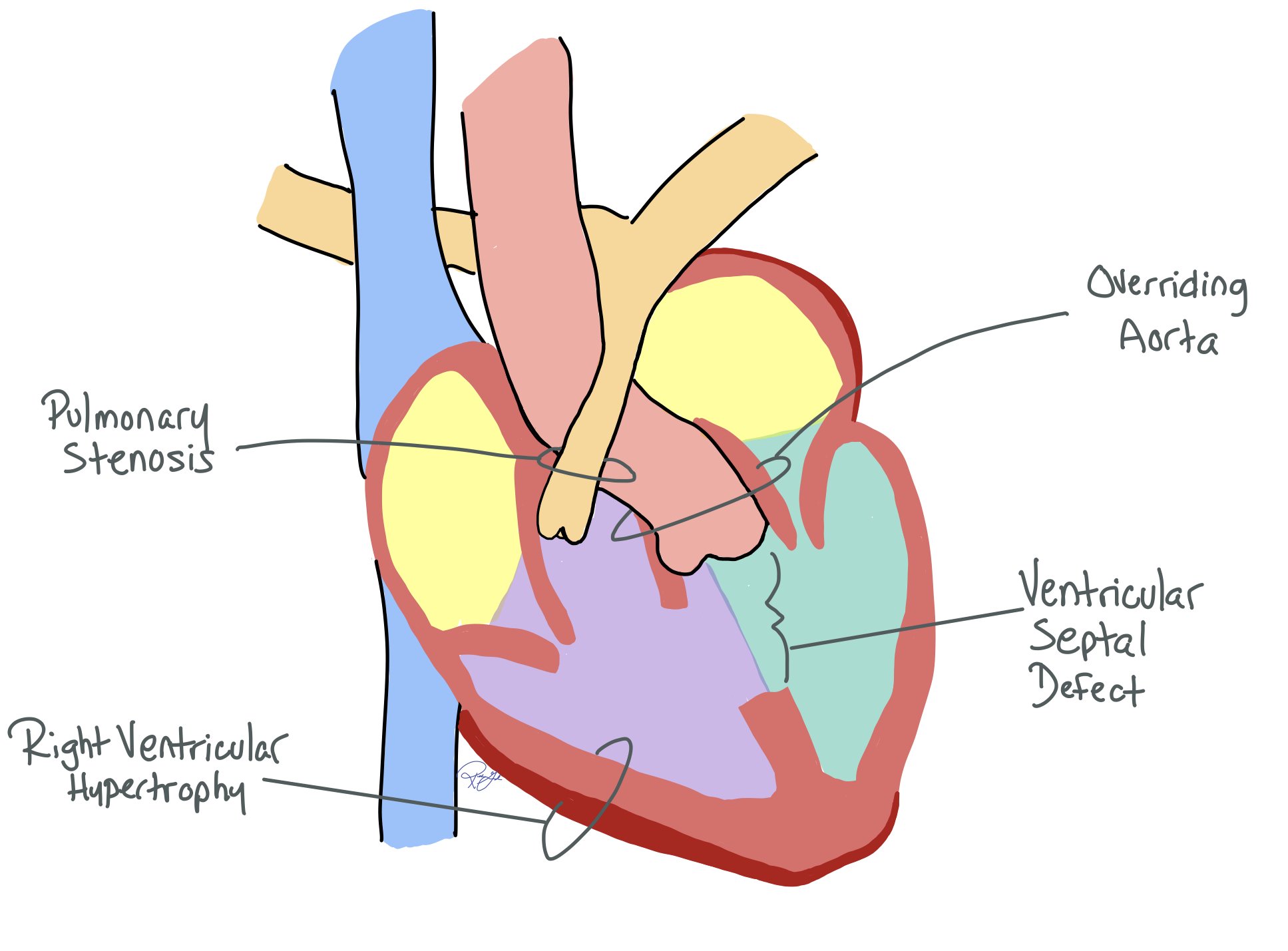
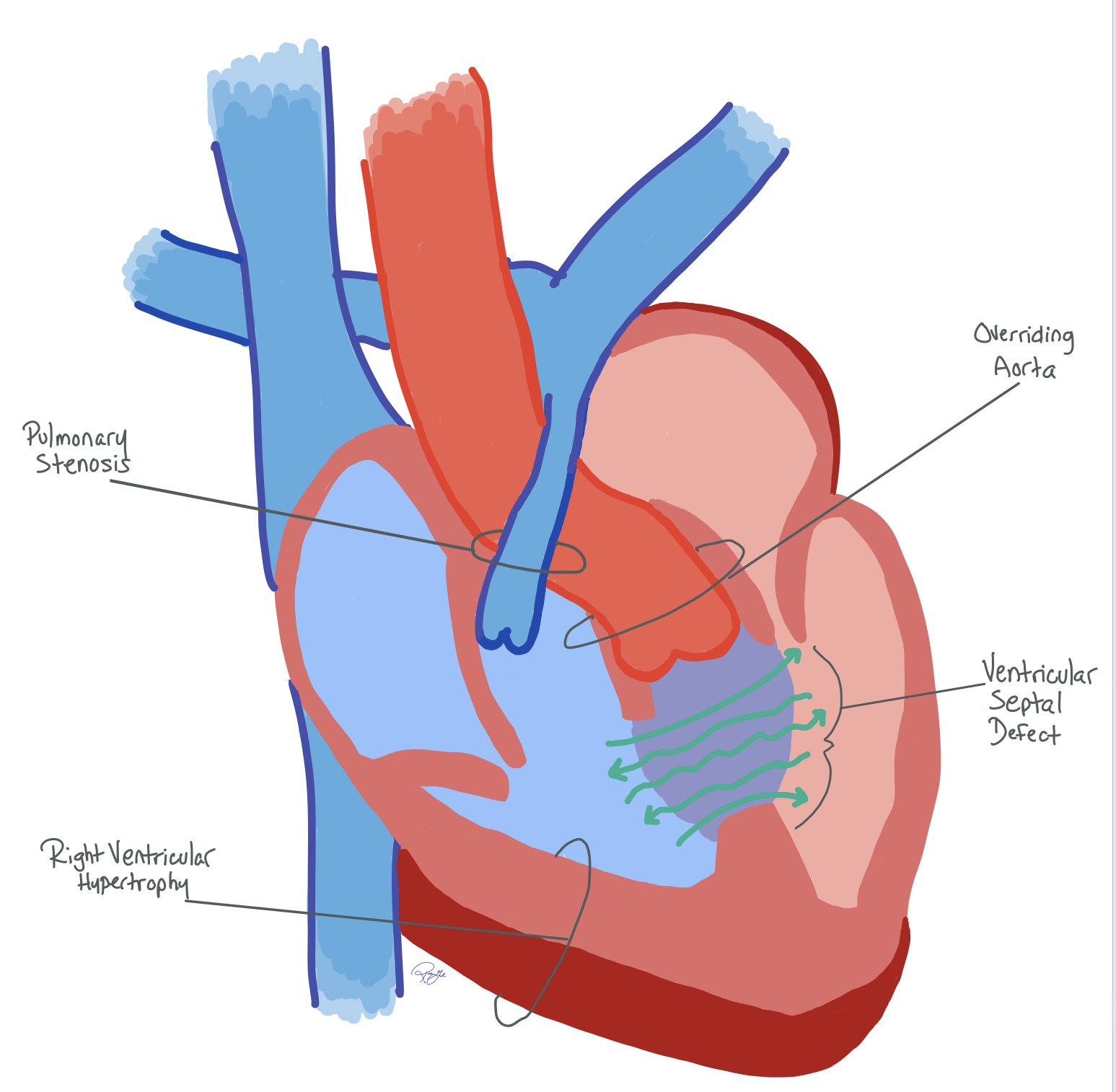
VSD: Anterior deviation of the infundibular septum creates a VSD, which is usually a single, large, subaortic defect. Since the VSD is unrestrictive, LV pressure = RV pressure
Overriding aorta: The aorta overrides the VSD instead of the LV due to the anterior deviation of the infundibular septum, allowing blood to enter from both ventricles
RVOT obstruction: The degree of RVOT obstruction can be variable and alter the resulting physiology and the complexity of the repair.
Minimal RVOT obstruction: Presents with similar physiology as a large VSD
RVOT obstruction without cyanosis “pink Tet”: If RVOT resistance is less than the aorta, shunting will occur L -> R and acyanotic
RVOT obstruction with cyanosis: If RVOT resistance is greater than the systemic resistance, shunting will occur R -> L and cause cyanosis. This will require surgery soon upon birth
Pulmonary Atresia: All pulmonary blood flow occurs through a PDA or major aortopulmonary collateral arteries requiring a complex repair with a right ventricle to pulmonary artery conduit (RV-PA conduit).
Multilevel obstruction: Obstruction can also occur at other levels:
Pulmonary valve can be stenotic and often bicuspid
Left pulmonary artery stenosis is common. Isolated pulmonary artery stenosis occurs in as many as 10% of cases
RV Hypertrophy: Increased pressure from the obstruction results in RV hypertrophy and increased systemic pressure. RVOT resistance can increase over time due to RV hypertrophy and worsening stenosis. This can lead to increased heart strain and RBBB on the EKG.
Various associated cardiac anomalies are common and can be in as many as 40% of patients. Some include abnormal coronary arteries, collateral aorticopulmonary vessels, PDA, and various endocardial cushion defects
Residual Features Post-repair: Many common lesions persist or develop post-repair resulting in complications in adulthood. Some include residual RVOT obstruction, persistent VSD, tricuspid regurgitation, pulmonary regurgitation, RVOT aneurysms, myocardial fibrosis, and remodeling
This deviation helps create the TOF pathophysiology, which is defined by:
Ventricular septal defect (VSD)
Aortic root overriding the VSD
Right ventricular outflow tract (RVOT) obstruction
Right ventricular hypertrophy
Clinical Features After Repair and Into Adulthood…
Often asymptomatic early in life following initial surgery. Residual lesions may be well tolerated in childhood but can progressively worsen over time.
Clinical signs and symptoms of right heart failure can present (exercise intolerance, shortness of breath, elevated JVP, ascites, peripheral edema), as well as various murmurs depending on the presence of residual defects or underlying pathophysiology.
Various atrial and ventricular tachyarrhythmias are common, presenting as palpitations or syncopal episodes. Ventricular tachycardia is a common cause of sudden cardiac death in patients following surgical correction.
Physical Exam Findings of TOF
Management
Initial Surgical Repai
TOF is the most common repaired cyanotic CHD in adults. Without surgery, half of TOF patients die within their first few years of childhood, and most do not live beyond 30 years of age.
In the United States, most patients with TOF undergo complete surgical repair in the first year of life. The goal of surgery is to separate pulmonary and systemic circulation while relieving the RVOT obstruction and maintaining RV function
Surgery comes with risks of perioperative complications or residual lesions, such as pulmonary regurgitation or residual obstruction.
The picture above is a visual of various potential repairs seen in those with TOF. Note: the pulmonary valve replacement surgery usually occurs 10-20 years after initial repairs.
Long-Term Complications In Adulthood:
25-year survival among repaired TOF is 93%. Arrhythmias and heart failure are the most common causes of late death.
Pulmonary regurgitation (PR): Very common following TOF repair and contributes heavily to the development of other life-threatening complications. It is most severe in transannular patch repairs
RV dysfunction: RV remodeling is associated with compensation for PR. Volume overload over time leads to dilation and myocardial fibrosis, as well as an increased risk of ventricular arrhythmias and SCD. RV volume is an important indicator for pulmonary valve repair
Ventricular fibrosis: RVOT fibrosis is nearly universal in adults following repair. Some degree of LV fibrosis is also common in over half of cases.
Aortic root dilation: likely secondary to increased volume load on the developing aorta in fetal life that can eventually result in aortic insufficiency.
Arrhythmias: Ventricular arrhythmias are a common complication and can result in sudden cardiac death. Many times this occurs in patients with TOF due to scar and fibrosis from the original surgery or from ventricular dilation and dysfunction.
Endocarditis: Increased risk for endocarditis following pulmonary valve replacement and repairs requiring conduits (i.e. anatomy with pulmonary atresia).
Pulmonary Valve Replacement and Indications:
Most adults with repaired TOF require pulmonary valve replacements at some point
This can be done surgically or percutaneously depending on the indication for valve repair and prior surgical history
Per the American College of Cardiology 2018 Guidelines for Pulmonary Valve Replacement:
Valve replacement is recommended in any patient with:
moderate or worse PR that is symptomatic
Sustained tachyarrhythmias
Residual lesions requiring surgical intervention
Valve replacement is indicated in asymptomatic patients with any two of the following:
Progressive decrease in exercise tolerance documented by exercise stress test
Mild to moderate RV or LV systolic dysfunction
Severe RV dilation (RV end-diastolic volume 160-180 ms/m2 measured via cardiac MRI)
Elevated RV systolic pressure >⅔ systemic pressure
Additional considerations include
QRS duration > 180 milliseconds or a rapid increase in a short period
Biventricular dysfunction
Moderate to severe TR
Long-Term Follow-Up
Other Long-Term Management and Follow-Up In Adulthood:
Annual follow-up with an adult congenital heart disease specialist has shown to improve outcomes in patients with complex congenital heart disease. These encounters focus on lifestyle management, symptoms of underlying cardiac pathology (palpitations, signs of heart failure, new murmurs), and routine monitoring
All patients should receive annual ECG and TTEs, a cardiac MRI every 1-3 years, and exercise testing every 3-4 years
Adults with TOF commonly require ICD or ablations for the management of arrhythmias. Per the AHA/ACC guidelines, ICDs should be considered in patients who meet standard criteria (LVEF <35%, NHYA class II or III symptoms) but there may be a role for ICDs for primary prevention in TOF patients who have additional risk factors
Spontaneous bacterial endocarditis prophylaxis for various procedures, including dental cleanings
Prophylaxis for life if a prosthetic valve or conduit is used, there is a prior history of IE, or residual VSD or ASD
Prophylaxis for the first 6 months after any valve replacement
Monitoring for risk factors of sudden cardiac death:
Increased risk is associated with LV systolic or diastolic dysfunction, non-sustained VT, QRS duration >180 ms, inducible sustained VT on exercise stress, and extensive RV fibrosis seen on cardiac MR
Family Planning Considerations and Pregnancy Complications:
Up to 10% of people who get pregnant following ToF repair have cardiac complications, most commonly arrhythmia. Complication risks increase substantially if significant PR is present
Offspring complications are rare but include higher rates of intrauterine growth restriction and an increased likelihood of having a congenital heart defect
All patients with TOF should be offered counseling on genetics, fetal screening, pregnancy risks, and contraception options
Back to the Case:
1. What further workup is needed to determine this patient's diagnosis and next steps in management?
Cardiac MRI is the gold standard tool to assess RV size and function. The RV end-diastolic volume is an important measurement in guiding decisions for pulmonary valve replacement. This patient has several risk factors for tachyarrhythmias given her clinical presentation and objective data (QRS >180 ms, TR, PR, elevated RV volume). An EP study with possible ablation and ICD placement should be strongly considered. Cardiac catheterization may also be indicated to have a better understanding of this patient's hemodynamics
2. What are long-term complications that can arise in adult patients with surgically correct Tetralogy of Fallot?
Pulmonic valve dysfunction, RV dilation, fibrosis leading to right heart failure, tachyarrhythmias and sudden cardiac death are all common long-term complications following Tetralogy of Fallot repair. Patients who hope to be pregnant should have preconception counseling to discuss the risks of maternal and offspring sequelae of ToF and have appropriate support for long-term management
3. What sort of preventative healthcare could have improved this patient's presentation?
Annual follow-up with an adult congenital heart disease specialist with regular imaging to assess disease progression. Regular TTE, ECG, cardiac MR, and exercise stress testing are all useful tools that can catch and prevent complications of Tetralogy of Fallot repair.
Further Learning:
Resident Responsibilities:
Be cognizant of including congenital heart disease in your differential in a relatively young patient with heart failure presentation. Also, don’t forget the basics of a complete H&P and take a thorough past medical and surgical history
Be able to identify the next step in imaging that may be helpful in determining future management. In addition to an ECG, TTE, and BNP, a cardiac MRI can be an important tool in guiding management in patients with TOF.
Given the high prevalence and risk of tachyarrythmias and sudden cardiac death in TOF patients, loop-in electrophysiologists early. Consider obtaining an EP study or ICD placement
How’d we do?
The following individuals contributed to this topic: Pal Shah, MD, Paul Cooper, MD, Peter Varga, MD
Chapter Resources
Alborikan, S., et al. (2021). "Blood biomarkers in patients with repaired Tetralogy of Fallot (rTOF); A systematic review and meta-analysis." International Journal of Cardiology Congenital Heart Disease 6: 100237.
Alkashkari W, Al-Husayni F, Almaqati A, AlRahimi J, Albugami S. An Adult Patient With a Tetralogy of Fallot Case. Cureus. 2020 Nov 23;12(11):e11658. doi: 10.7759/cureus.11658. PMID: 33391897; PMCID: PMC7769492.
Bokma, J. P., et al. (2023). "Improved Outcomes After Pulmonary Valve Replacement in Repaired Tetralogy of Fallot." Journal of the American College of Cardiology 81(21): 2075-2085.
Downing TE, Kim YY. Tetralogy of Fallot: General Principles of Management. Cardiol Clin. 2015 Nov;33(4):531-41, vii-viii. doi: 10.1016/j.ccl.2015.07.002. Epub 2015 Aug 29. PMID: 26471818.
Doyle T, Kavanaugh-McHugh A. Tetralogy of Fallot (TOF): Management and outcome. In: UpToDate, Connor RF (Ed), Wolters Kluwer. (Accessed on July 20, 2024.)
Doyle T, Kavanaugh-McHugh A. Tetralogy of Fallot (TOF): pathophysiology, clinical features, and diagnosis. In: UpToDate, Connor RF (Ed), Wolters Kluwer. (Accessed on July 20, 2024.)
Fish F, Weingarten, A. Tetralogy of Fallot (TOF): Long-term Complications and follow-up after repair. In: UpToDate, Connor RF (Ed), Wolters Kluwer. (Accessed on July 20, 2024.)
Piazza L, Chessa M, Giamberti A, Bussadori CM, Butera G, Negura DG, Micheletti A, Callus E, Carminati M. Timing of pulmonary valve replacement after tetralogy of Fallot repair. Expert Rev Cardiovasc Ther. 2012 Jul;10(7):917-23. doi: 10.1586/erc.12.67. PMID: 22908924.
Possner, M., et al. (2020). "Risk Factors for Mortality and Ventricular Tachycardia in Patients With Repaired Tetralogy of Fallot: A Systematic Review and Meta-analysis." Canadian Journal of Cardiology 36(11): 1815-1825.
Stout, K. K., et al. (2019). "2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines." Circulation139(14): e698-e800.