Case Presentation:
A 70 year old male with PMH of DM, HTN, and HLD presents to the hospital with chest pain found to be in cardiogenic shock. Right before he goes to the cath lab, the patient goes into VT arrest. A number one emergency is called with quick return to sinus rhythm after defibrillation. He is intubated and brought immediately to the cath lab, where he was found to have a 100% occlusion of his LAD. He comes back to the floor post stent placement on a dobutamine drip and with an Impella mechanical circulatory support device for left sided support. You are standing outside his ICU room when you hear the Impella console start to alarm.
Ask Yourself:
Questions:
1. What are the indications for mechanical support?
2. What is the difference between a balloon pump and an Impella? When do we use them?
3. How do you read the device screens?
Types of Mechanical Support Devices
In this review, we will discuss two main types of mechanical support devices.
The first is the Balloon Pump, which is positioned below the subclavian artery and above the renal artery (Figure 2). The balloon pump uses the inflation and deflation of a balloon to help increase coronary perfusion, decrease cardiac work, and increase cardiac output.
The second is the Impella, which sits in the left ventricle (Figure 3). The Impella uses a motor to help pull blood out from the left ventricle into the aorta. This helps augment cardiac output and decrease cardiac work.
Note: Impellas require therapeutic anticoagulation while Balloon Pumps do not.
The Balloon Pump:
The Balloon Pump uses counterpulsation to help enhance cardiac function. It is positioned a few centimeters below the subclavian artery and above the renal arteries.
The balloon inflates during diastole, which helps augment diastolic pressure. This will help with retrograde flow into the coronary arteries, thus helping with coronary filling and myocardial perfusion. Plus, since the heart has more time to fill, this will also help with increasing systemic arterial pressure.
The balloon will deflate right before the aortic valve opens for systole. This creates a vacuum in the aortic valve, which helps to pull the blood out of the heart and decrease both afterload and work of the heart. This ultimately helps to decrease myocardial demand and allows the heart to not have to work as hard.
Considerations:
In order to use a balloon pump, the LV must still retain some function
The balloon must be placed below the subclavian artery and above the renal arteries. If the balloon migrates too far up or down, the patient is at risk for perfusion deficits to the brain, upper extremity, or kidneys. Therefore, make sure you get daily CXR to assess placement (Figure 4)! Also make sure that you are frequently assessing both upper and lower extremity pulses to ensure good perfusion.
Uses:
Myocardial infarctions with cardiac output impairment
Cardiogenic shock
Heart failure with poor cardiac output
High risk PCI
Weaning from bypass
LV offload while on VA ECMO
Contraindications:
Aortic Insufficiency or regurgitation
Aortic dissection or aneurysm
Severe peripheral artery disease (this can impede perfusion)
Sepsis (you don’t want anything in the body that can act as a nidus of infection)
People who cannot be anticoagulated as patients on balloon pumps need to be therapeutically anticoagulated.
Figure 4A shows a CXR of a patient with both a Balloon Pump and Swan Catheter. Figure 4B outlines specifically where the Balloon Pump (green) and Swan Catheter (gold) are. Finding these two landmarks can be difficult due to other wires and lines, such as external telemetry boxes, on top of the patient. Note how the top of the Balloon Pump is seen at the aortic notch ~2cm away from the subclavian!
Figure 5 shows a picture of the Balloon Pump screen. On the top, you can see the patient’s heart rate (green). Directly below that, you can see the arterial line tracing from the aorta and the mean arterial blood pressure (red). In this picture, the balloon has a 1:2 augmentation, meaning that every other beat is augmented by the balloon. On the bottom line, you can see the balloon pump tracing (blue) and the augmentation in grey, which signifies how much the balloon pump is actually supporting the patient.
In order to properly understand the balloon pump screen, you must understand what is triggering the balloon pump to inflate or deflate:
→ The EKG Tracing. Here, the balloon pump will time its inflation and deflation with the cardiac cycle based upon the EKG tracing. You can decide to augment every beat (1:1), every other beat (1:2), or every third beat (1:3).Setting the pump to 1:2 or 1:3 allows for you to see augmentation variability (Figure 5).
→ Arterial Blood Pressure Waveform. This uses the arterial line from the balloon pump to measure the intra-aortic pressures. The changes in pressures allow the pump to know where the patient is in the cardiac cycle and thus when to inflate or deflate. In patients who may have irregular cardiac cycles as in atrial fibrillation, this is a great option as it can account for cycle variability
Note: Some consoles have built in systems for an atrial fibrillation trigger, which will analyze the QRS complexes and time the balloon to deflate when sensing the R wave. There are even paced triggers for patients who are ventricularly paced.
Balloon Pump Waveforms Explained:
Figure 6 shows both the normal arterial pressure tracing (seen in red). Each cycle starts at the end of diastole, which is the lowest pressure in the system, and begins with the opening of the aortic valve. When the aortic valve opens, the pressure in the system increases until it reaches peak systolic pressure. This represents systole (depicted by the green area under the curve). The closing of the aortic valve, slightly increases the pressure in the system, which can be seen via the Dicrotic Notch. Diastole begins with the closing of the aortic valve and ends prior to the aortic valve reopening (as seen by the purple shaded area under the curve).
In Figure 7, we can see both the normal and augmented aortic pressure tracings (red) in conjunction with the pressure tracings of the balloon itself (blue). Note how the balloon inflates during diastole, causing an increased pressure in the system and augmenting the peak diastolic pressure. When the balloon deflates, it decreases the pressure in the system drastically. Since the balloon deflates right before the aortic valve reopens, it actually creates a negative pressure which helps increase cardiac output (CO) without increasing cardiac work.
The Impella:
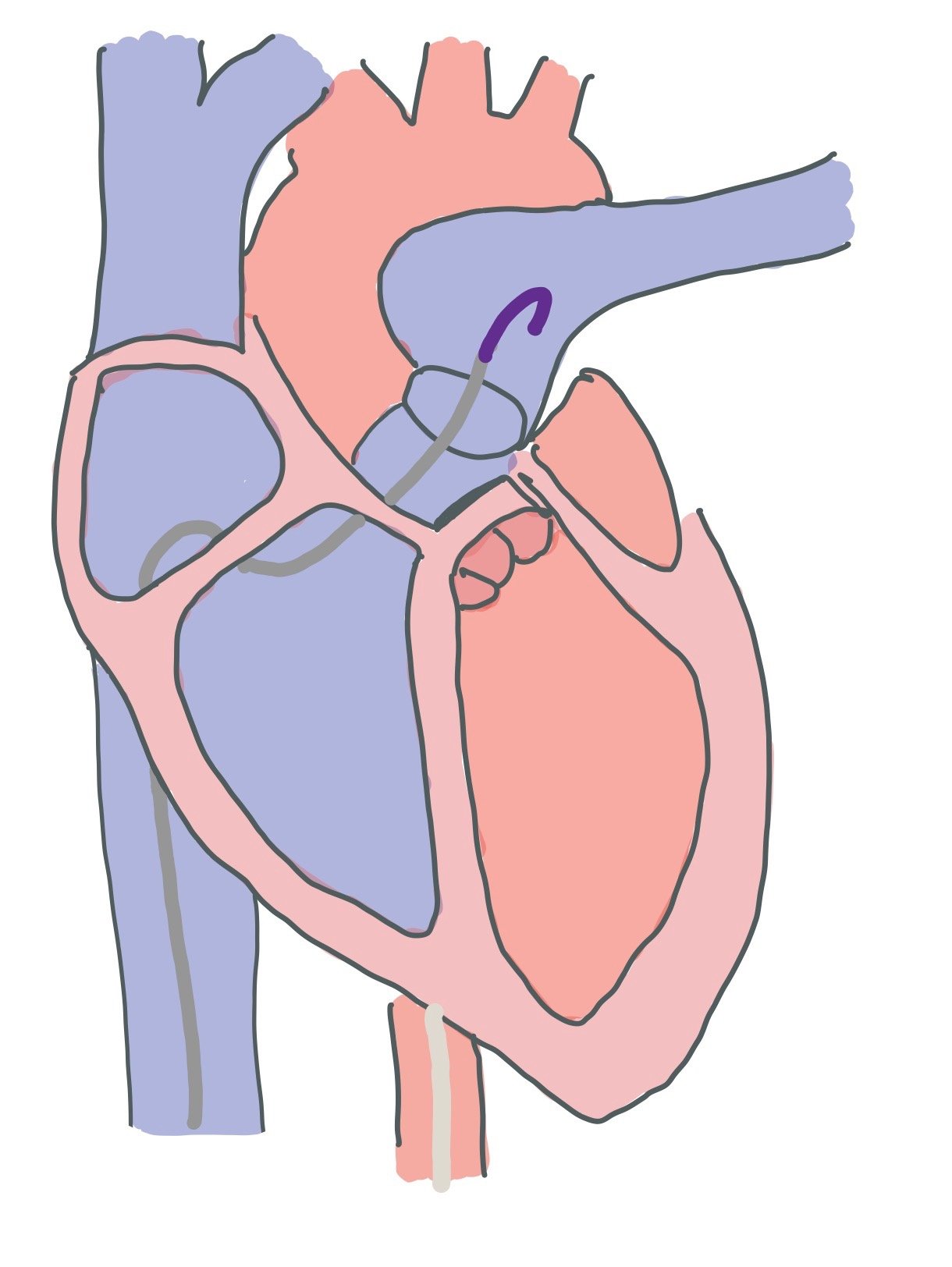
The Impella is a continuous axial flow motor that helps to pump blood from one place to the other. Most catheters have a pigtail at the end, the inlet where blood is pull from, the outlet where blood is pulled to, and a motor to help pump the blood (Figure 8). There is a purge solution that runs in the opposite direction of the blood that is made up of D5W and bicarbonate that prevents blood from clotting in the motor. While the purge solution used to be made up of heparin, manufacturers are moving away from this as it is easier to manage for nursing staff to manage.
While we mainly use a left sided Impella which sits in the left ventricle and helps pump blood from the left ventricle to the aorta, there is also a right ventricular Impella that brings blood from the inferior vena cava to the pulmonary arteries. When an Impella is in the left ventricle, you can do a bedside ultrasound to confirm placement of the device (Figure 8). If the device migrated too far distal into the LV, you may get a suction alarm if the device hits the ventricular wall!
Although the Impella has a continuous flow and works in both systole and diastole, we titrate therapy to the MAPs. This is because the systolic pressures may remain the same as the Impella is helping to offload the ventricle, while the diastolic pressure will increase due to the augmentation of the diastolic flow and increase in the diastolic pressure (thus increasing MAP). The Impella is named by how many liters of blood flow that it provides (i.e. the Impella 2.5 that only provides 2.5L/min vs the Impella 5.5 that provides 5.5L/min).
Note! The Impella is preload dependent. If there is not enough preload (i.e. RV failure, distributive shock, bleeding, etc), this can create a suction alarm. If this happens, giving fluid boluses can help. Thus, target central venous pressure (CVP) should be >10. If patients do have RV failure, it is important to recognize this quickly as they may require inotropes, right sided Impella, or ECMO.
Contraindications: mural thrombus, presence of a mechanical aortic valve, aortic valve stenosis/calcification, aortic insufficiency, severe peripheral arterial disease, combined cardiorespiratory failure, Atrial/Ventricular Septal Defect, Left ventricular rupture, and Cardiac tamponade.
Figure 8 is a drawn picture of a parasternal long bedside POCUS view. In this view, you can see positioning of the Impella. Specifically, you are looking to ensure that the pigtail part of the catheter is not hitting the wall and that the inlet of the catheter (where blood is pull from) is ~3.5cm from the aortic valve annulus.
Figure 9A depicts a CXR of a patient with both an Impella and a Swan Catheter. 9B outlines the Impella (pink) and Swan catheter (gold).
The Impella Screen:
Figure 10 is a drawn image of the Impella screen. Starting with the waveforms, the red line represents the aortic pressure signal, while the white line represents the ventricular pressure signal that the device estimates. When mispositioned, these placement signals will change. The green line represents the current of the device. When functioning properly, this line should be pulsatile, peaking when the aortic valve is open. A flat line indicates the device may have drifted.
The top left has an Alarm section, which will turn red (indicating no Impella flow), yellow (suboptical Impella flow), or white (simply informational). To the right of the alarm section, there is a Suggestion Box, which will give you a step by step guide on how to fix whatever it is that is causing the machine to alarm.
On the bottom, you can see Impella flow, which will give both the max, min, and average flow. The section next to that is the Purge System, which focuses on the the flow and pressure of the purge fluid that helps the Impella run and prevents it from clotting. The section to the right gives estimated Cardiac Output (CO) and Cardiac Power Output ([MAPxCO]/451), allowing us to see how well the Impella is helping with body perfusion. The dials to the right of the machine include the Mute Alarm, Flow Control, Display, Purge Menu, and Menu buttons. These control how the Impella works and where you go to make device adjustments. These should only be adjusted under the direction of the attendings or fellows.
Note! Cardiac Power Output (CPO) is the #1 indicator of in hospital mortality when <.53. Therefore, our goal is >.6!
The Right Sided Impella:
Recently, an Impella was approved to help augment the right side of the heart. Specifically, the Impella, AKA the Impella RP, helps to pump blood from the IVC to the pulmonary artery. This device is approved for use up to 14 days and can be used on its own for right sided heart failure or in conjunction with a left sided Impella to offer biventricular support when modalities such as ECMO are unavailable.
Contraindications: Mechanical valves, severe valvular stenosis or regurgitation of the tricuspid or pulmonary valves, IVC filter, right atrial or vena cava mural thrombus
Adverse Side Effects: Side effects include arrhythmias, tamponade, vascular injury, cardiogenic shock, hepatic failure, pulmonary valve insufficiency, infection, and tricuspid valve injury
Right sided Impella, AKA the Impella RP. It goes from the inferior vena cava (IVC) to the pulmonary artery and helps to augment blood flow through the right side of the heart
In the second picture, you can see both a right and left sided Impella. Together, these two devices offer biventricular support.
Back to the Case:
1. What are the indications for mechanical support?
Mechanical circulatory support should be considered in patients with clinical and biochemical signs and symptoms of hypoperfusion secondary to cardiogenic shock when there is an inadequate hemodynamic response of medical pharmacotherapy/inotrope support.
2. What is the difference between a balloon pump and an Impella? When do we use them?
The IABP assists the heart indirectly by decreasing the afterload and augments diastolic aortic pressure. The device is used for a few days in typical circumstances, though there are reports of use up to 30 days. IABP has minimal effects on preload and cannot independently support the systemic circulation in cases of cardiogenic shock.
The Impella actively unloads the left ventricle and provides up to 5.5 L/min of flow. The device is approved for up to 10 days of use. The device is approved for use in cardiogenic shock following acute myocardial infarction, surgery, cardiomyopathies, or myocarditis that is not responding to conventional medical therapies. It may be used in high-risk percutaneous coronary intervention as a means of preventing hemodynamic instability.
3. How do you respond when the device alarms?
It’s important to familiarize yourself with the device and its screen. Identify the type of alarm and check vitals. The type of alarm will give a significant amount of information. If the alarms do not identify the problem, investigate the waveforms. Is the green line flat? Have the placement waveforms changed? If yes, the device has likely migrated. First contact your fellow or attending and explain what you’re seeing. Basic steps to addressing the problem include checking to make sure the line itself has not gotten kinked. The first step is to get a bedside POCUS of a parasternal long to locate the Impella. Next step would be to get a stat CXR, although this would take longer and might not give you all the information you need. Decrease the device flow via the flow control from setting P9 to setting P2 until the issue is resolved (with permission from a fellow or attending). Further adjustments may need to be made with more staff available for support.
Further Learning:
Resident Responsibilities
-If you have a patient on a mechanical support device in the ICU, take time to familiarize yourself with the screen and operations
Make sure to assess distal pulses regularly! It is important to get a baseline and periodic physical exam. Lack of pulses can mean device migration or hematoma formation
Look out for vascular complications, such as hematoma formation or lack of distal pulses,
Be able to read and understand the device screen for either an IABP or an Impella device
If you are concerned about device placement, do a bedside echo!!
Make sure to get daily CXR to assess device placement
Attending and Fellow Pearls
If an alarm sounds, respond promptly by looking at the screen to see why the device is alarming.
-Recognize when to escalate to a fellow or attending and be able to accurately information from the device
Looking at the balloon augmentation, overall cardiac output, and MAP will allow you to determine whether or not the device is working correctly. Make sure to look at the waveform
Further Reading
Review of the different types of mechanical support devices:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9524665/
Review of Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support:
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.040654
How’d we do?
The following individuals contributed to this topic: Brandon Bedell, MD, Amer Ardati, MD
Chapter Resources
1. Automated Impella ControllerTM Overview | HeartRecovery.com. Accessed April 26, 2023. https://www.heartrecovery.com/education/education-library/qsv-automated-impella-controller-overview
2. Ergle K, Parto P, Krim SR. Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients With Acute Cardiogenic Shock. Ochsner J. 2016;16(3):243-249.
3. Glazier JJ, Kaki A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int J Angiol Off Publ Int Coll Angiol Inc. 2019;28(2):118-123. doi:10.1055/s-0038-1676369
4. Schrage B, Becher PM, Bernhardt A, et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation. Circulation. 2020;142(22):2095-2106. doi:10.1161/CIRCULATIONAHA.120.048792
5. Intra-Aortic Balloon Pump (IABP) Implant. Center for Advanced Cardiac and Vascular Interventions. Accessed April 29, 2023. https://cacvi.org/services/cardiac-procedures/intra-aortic-balloon-pump-implant/
6. Khan MZ. Impella Device is Superior to an Intra-Aortic Balloon Pump in Cardiogenic Shock. Int Arch Cardiovasc Dis. 2021;5(2):040. doi:10.23937/2643-3966/1710040
7. Khan TM, Siddiqui AH. Intra-Aortic Balloon Pump. In: StatPearls. StatPearls Publishing; 2023. Accessed April 30, 2023. http://www.ncbi.nlm.nih.gov/books/NBK542233/
8. Safety Information | Abiomed.com. Accessed April 27, 2023. https://www.abiomed.com/important-safety-information
9. Satish Mishra M.D., M.S. Short Term Mechanical Support.
10. Impella Heart Pump - Critical Care - allnurses. Accessed April 26, 2023. https://allnurses.com/impella-heart-pump-t740606/
11. Balthazar, T. M.D.., Vandenbriele, C., M.D., PhD. Part 9 Impella Troubleshooting and Resuscitation. In: Editor AA, Editor BB, eds. ACVC Handbook MCS 2022. European Society of Cardiology
12. Pucher PH, Cummings IG, Shipolini AR, McCormack DJ. Is heparin needed for patients with an intra-aortic balloon pump? Interact Cardiovasc Thorac Surg. 2012 Jul;15(1):136-9. doi: 10.1093/icvts/ivs017. Epub 2012 Apr 11. PMID: 22495506; PMCID: PMC3380966.
13. Atti V, Narayanan MA, Patel B, Balla S, Siddique A, Lundgren S, Velagapudi P. A Comprehensive Review of Mechanical Circulatory Support Devices. Heart Int. 2022 Mar 4;16(1):37-48. doi: 10.17925/HI.2022.16.1.37. PMID: 36275352; PMCID: PMC9524665.
14. Taleb, Iosif, et al. “Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support: A Proof of Concept.” Circulation (New York, N.Y.), vol. 140, no. 1, 2019, pp. 98–100, https://doi.org/10.1161/CIRCULATIONAHA.119.040654.